
Nestlé Health Science presents new data showing HMO blend is safe and effective in infants with severe cow’s milk protein allergy
Nestlé Health Science presents first data at the EAACI Hybrid 2021 Congress from the PLATYPUS study, a key study in its breakthrough clinical trial program evaluating the safety and benefits of Nestle’s breastmilk-identical Human Milk Oligosaccharides blend (HMO;2’FL and LNnT) in infants and young children with cow’s milk protein allergy (CMPA).
The data presented showed that the amino acid-based formula, Alfamino® HMO, following the addition of Nestle’s HMO blend, is safe, promotes normal growth and is effective specifically for those with moderate-severe CMPA, a particularly vulnerable patient population.1
Data already presented from studies in the same clinical trial program (IVORY and CINNAMON) showed that Althéra® HMO, an extensively hydrolysed formula supplemented with the same HMO, is safe, hypoallergenic, reduces the risk of respiratory tract infections, positively shapes the gut microbiome and supports normal growth in the wider population of infants with CMPA.2,3,4,5
These studies also corroborate findings from another Nestlé-sponsored clinical trial with healthy infants fed a standard infant formula containing the same HMO blend, where it was shown to shift the gut microbiota closer to that of healthy breastfed infants, which was linked to fewer lower respiratory tract infections and lower use of antibiotics.6,7
HMO are the third largest solid component in breast milk.8 While protective benefits of HMO have been recognized for several decades, the production of breast milk-identical HMO has only recently become possible.8 2’FL and LNnT are two of the most significant HMO in breast milk.8
HMO are potentially valuable components to support the most vulnerable with CMPA which is an immune-mediated disease. Symptoms may affect the digestive, skin, respiratory systems,9 but it’s less known that CMPA is also associated with increased gut permeability and a disturbed gastrointestinal microbiota (dysbiosis).10,11 This is thought to delay immune maturation and contribute to a higher risk for infections. 8,12-14
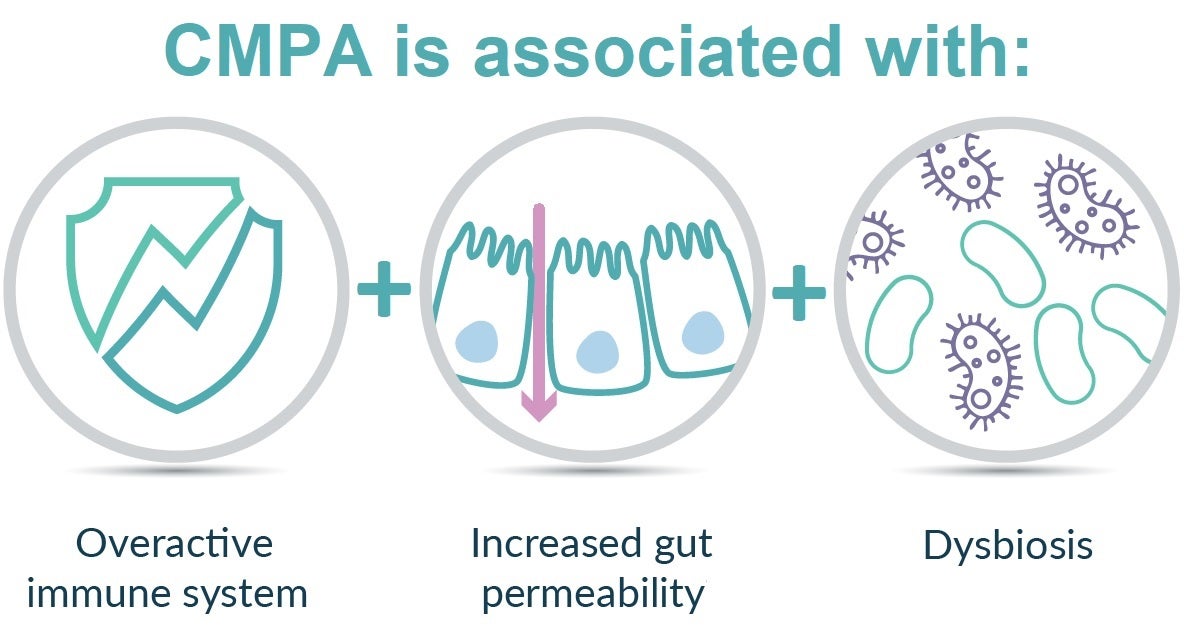
The PLATYPUS study, conducted in Australia, is the first to evaluate infants with moderate-severe CMPA being fed a hypoallergenic amino acid-based formula (AAF) supplemented with HMO.1 In addition, very few AAF are tested in the target moderate-severe CMPA patient population, which makes the PLATYPUS study unique.1
Professor Michael Gold, Department of Allergy and Clinical Immunology, University of Adelaide, Australia, the principal investigator from the PLATYPUS study said, “The addition of two HMO to an amino acid based formula prescribed for infants with moderate to severe CMPA was well tolerated and highlighted the safety of HMO in this patient population”. 1
Nestlé Health Science is committed to leading HMO research in infants with CMPA. It has commenced a global launch of the extensively hydrolyzed (Althéra® HMO and Alfaré® HMO) and amino acid-based (Alfamino® HMO) formula portfolio with 2’FL and LNnT for the management of infants with CMPA and/or multiple food allergies. Further data on the microbiome and metabolome from the CINNAMON and PLATYPUS studies is expected in 2021 and 2022.
IMPORTANT NOTICE: Mothers should be encouraged to continue breastfeeding even when their infants have cow’s milk protein allergy. This usually requires qualified dietary counseling to completely exclude all sources of cow’s milk protein from the mothers’ diet. If a decision to use a special formula intended for infants is taken, it is important to give instructions on correct preparation methods, emphasizing that unboiled water, unsterilized bottles, or incorrect dilution can all lead to illness. Formula for special medical purposes intended for infants must be used under medical supervision.
Nestlé Health Science is a leader in the science of nutrition and a globally managed business unit of Nestlé. We believe in empowering healthier lives through nutrition and are committed to redefining the management of health, offering an extensive portfolio of science-based active lifestyle nutrition, medical nutrition and pharmaceutical solutions. Our extensive research network, both within Nestlé’s R&D centers as well as with external partners, provides the foundation for products that can help people to live their healthiest lives. Headquartered in Switzerland, we have more than 7,000 employees around the world, with products available in more than 140 countries. www.nestlehealthscience.com
Media contact: Jacquelyn.Campo@nestle.com
2’fucosyl-lactose = 2’FL
Lacto-N-neotetraose = LNnT
- Gold M, et al Growth, tolerance and safety of an amino acid-based formula supplemented with two human milk oligosaccharides in infants with moderate-to-severe cow’s milk protein allergy. Abstract presented at EAACI Digital. July 10-12, 2021 (PLATYPUS Study)
- Nowak-Wegrzyn A et al. Confirmed hypoallergenicity of a novel whey-based extensively hydrolyzed infant formula containing two human milk oligosaccharides. Nutrients 2019;11:1447. (IVORY Study).
- Vandenplas Y, et al Growth tolerance and safety of an extensively hydrolyzed formula containing two human milk oligosaccharides in infants with cow’s milk protein allergy. Abstract presented at EAACI-PAAM, Florence, Italy. October 17-19, 2019 (CINNAMON Study).
- Vandenplas Y et al. Extensively hydrolysed formula with two human milk oligosaccharides reduces rate of upper respiratory tract infections in infants with cow’s milk allergy. Abstract presented at EAACI Digital. June 6-8, 2020 (CINNAMON Study).
- Pedersen HK and et al. An extensively hydrolysed formula supplemented with two human milk oligosaccharides (HMO) shapes the gut microbiome in infants with cow’s milk protein allergy. Abstract presented at EAACI FAAM October 2020 (CINNAMON Study).
- Puccio G et al. Effects of infant formula with human milk oligosaccharides on growth and morbidity: A randomized multicenter trial. J Pediatr Gastroenterol Nutr 2017;64:624-631.
- Berger B, et al. Linking Human Milk Oligosaccharides, Infant Fecal Community Types, and Later Risk To Require Antibiotics. mBio 2020;11:e03196-19.
- Human Milk Oligosaccharides, New Ways to Shape the Gut Microbiome in Cow’s Milk Protein Allergy. Nestlé Health Science Symposia Proceedings from EAACI 2019. Eur Med J Allergy Immunol 2019;4:48-54.
- Koletzo S et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr 2012;55:221-229
- Donovan SM and Comstock SS. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann Nutr Metab 2016;69(Suppl 2):42-51.
- Francavilla R et al. Effect of lactose on gut microbiota and metabolome of infants with cow's milk allergy. Pediatr Allergy Immunol 2012;23:420-427.
- Woicka-Kolejwa K et al. Food allergy is associated with recurrent respiratory tract infections during childhood. Postepy Dermatol Alergol 2016;33:109-13.
- Juntti H et al. Cow's milk allergy is associated with recurrent otitis media during childhood Acta Otolaryngol 1999;119:867-73
- Tikkanen S et al. Status of children with cow's milk allergy in infancy by 10 years of age Acta Paediatr 2000;89:1174-80.

