Advancing the management of cow’s milk protein allergy
Breast milk is the best nutrition for infants, not only providing nutrients for growth, but also bioactive components, like human milk oligosaccharides (HMO), that support the development of the microbiota and immune system. The supplementation of infant and hypoallergenic specialty formulas with novel structurally identical HMO (2’FL and LNnT) has been shown to prime the immune system of healthy infants and infants with cow’s milk protein allergy (CMPA).
The maturation of infant’s immune system
The first year of life is a critical phase during which the foundations of a child’s development are laid.1 This is a period of heightened vulnerability for infants, but also an important window of opportunity, to help shape their immune system as it matures.1-3 The intestinal tract, the gut microbiota and the immune system are closely interlinked.3 The gut microbiota promotes the development and education of the naïve immune system, and the immune system and microbiota promote the development of the intestinal tract.3,4
Breast milk supports the developing immune system. Breast milk is the gold standard in infant nutrition and it confers protection in early life.5-7 Infants exclusively breastfed for at least 4-6 months have a lower incidence of gastrointestinal, upper and lower respiratory tract infections compared to exclusively formula-fed infants.8 Breastfeeding may also be associated with a reduced risk of allergic disease, like asthma.9,10
The reduced rate of infections and allergies in breastfed infants is thought to be related to the immune-modulating and microbiome modifying effects of components such as lactose and human milk oligosaccharides.9,11
Human milk oligosaccharides
Breast milk has been shaped over the course of human evolution in order to provide tailor-made nutrition and protection to infants.12 Human milk is uniquely rich in complex predominantly nondigestible carbohydrates called HMO. The concentration of HMO in human milk is 100–1000 times higher compared to the milk of any domesticated farm animal.12
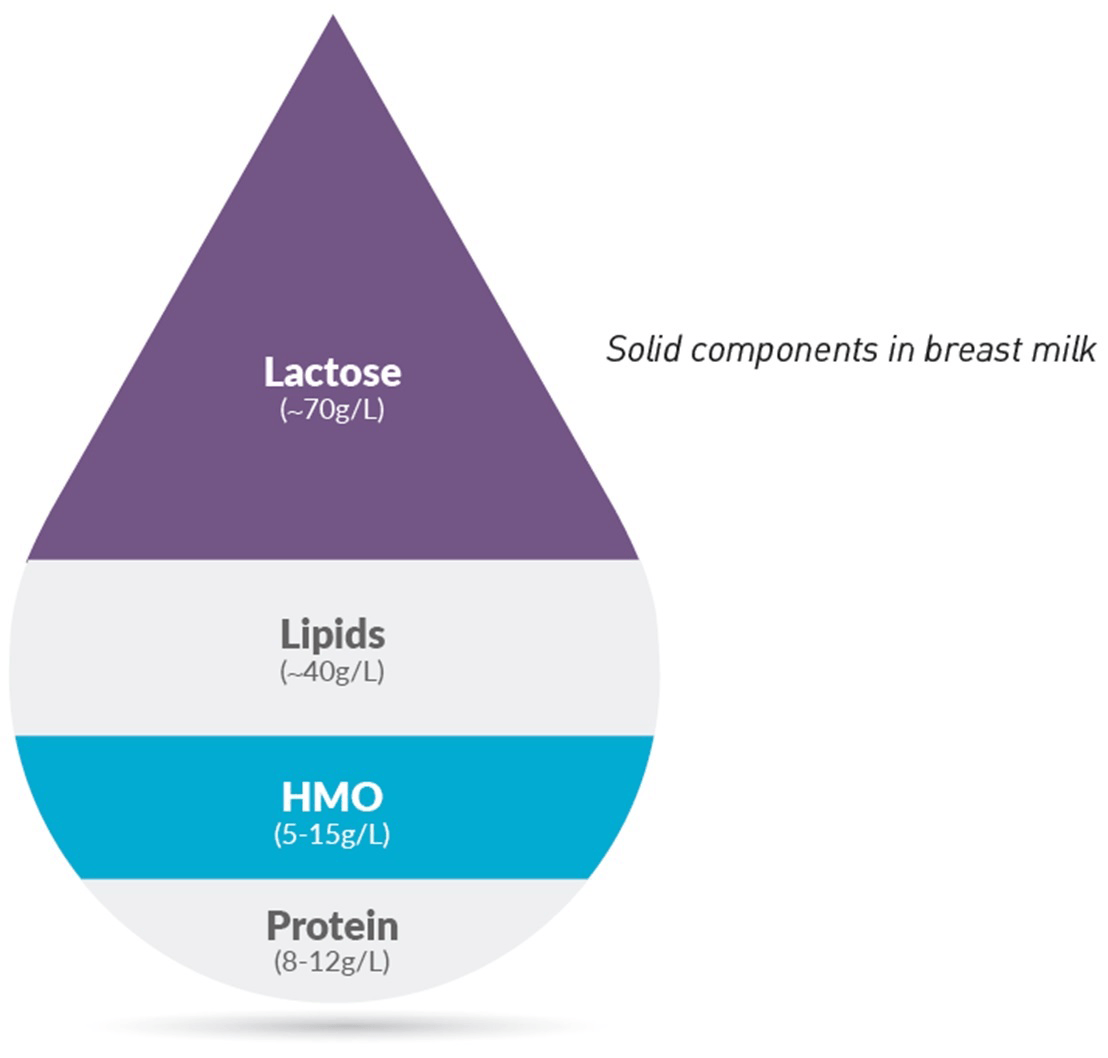
After lactose and lipids, HMO are the 3rd most abundant solid component of breast milk with over 200 unique HMO structures having been identified.12 Two of these HMO, 2’FL (2’-Fucosyllactose) and LNnT (Lacto-N-(neo)tetraose), typically account for more than 30% of HMO.13
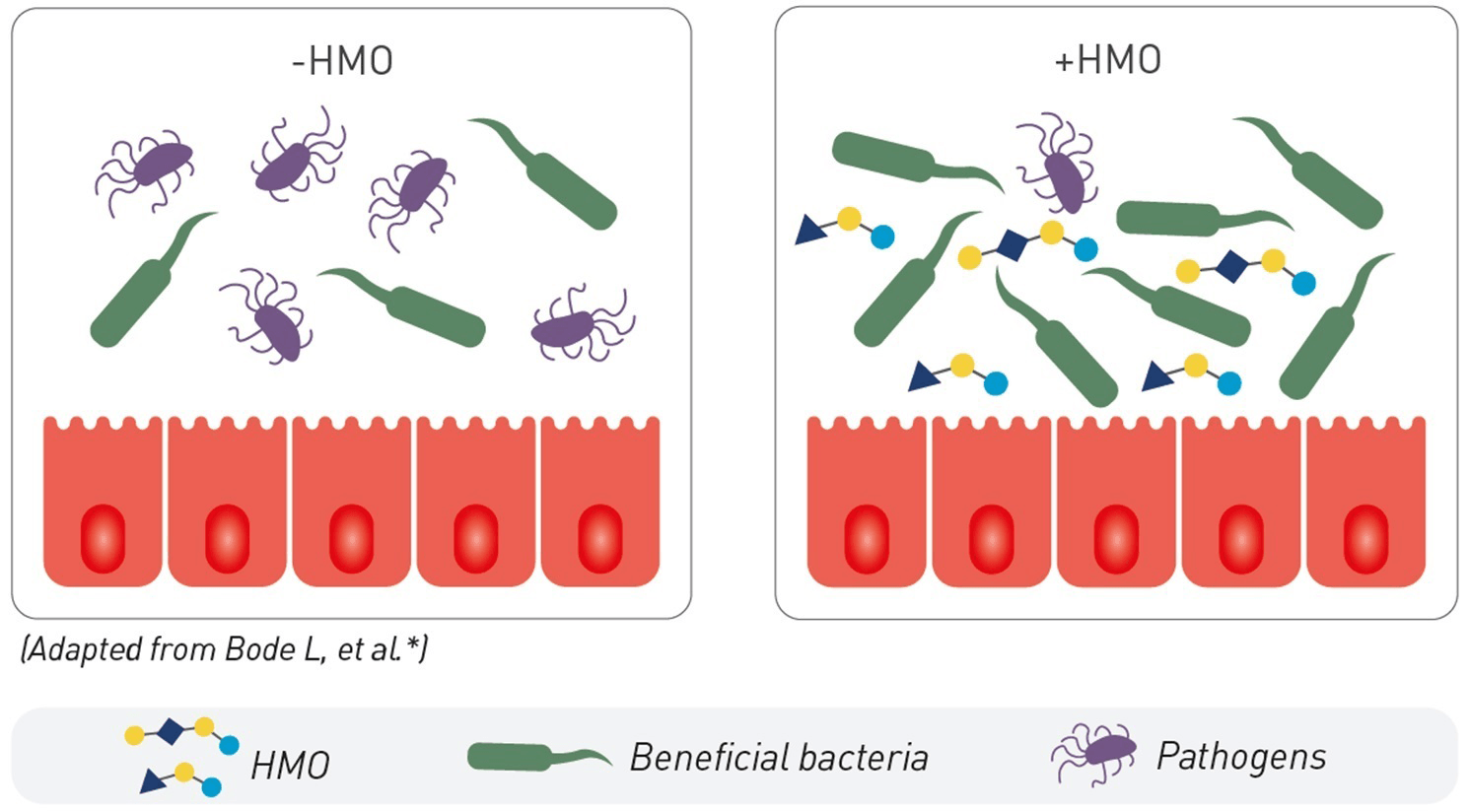
1. SUPPORT OF GUT MICROBIOTA
HMO promote the growth of beneficial bacteria that colonise the infant’s gut where about 70% of all immune cells reside.11 2’FL and LNnT have been shown to increase the abundance of bifidobacteria, which produce short chain fatty acids, that are critical for gut health.14
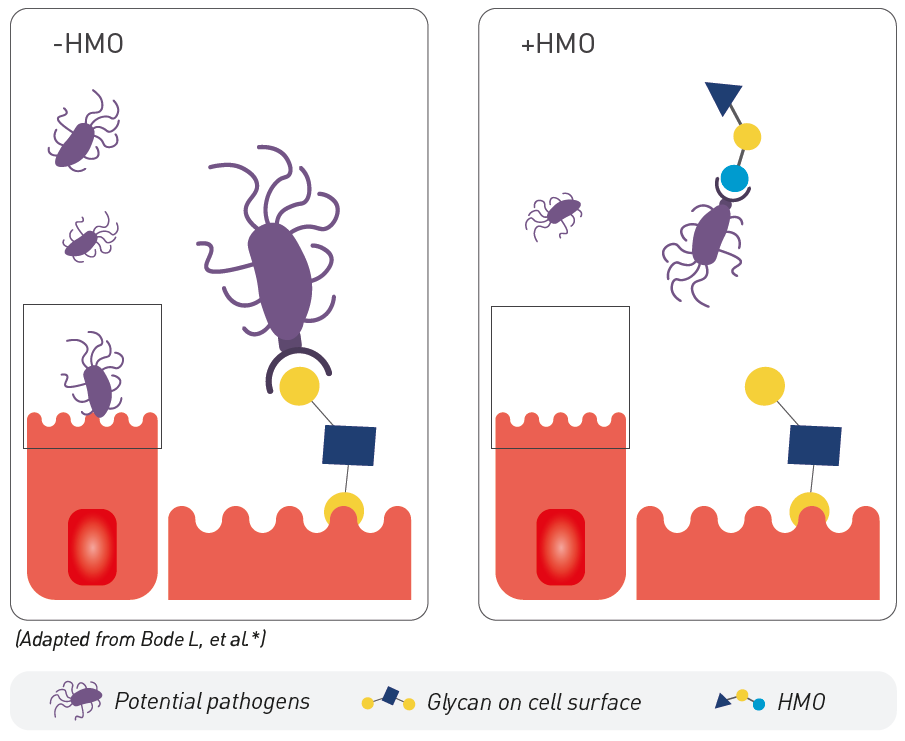
2. PATHOGEN ELIMINATION
HMO serve as soluble decoy receptors to prevent pathogen attachment to infant mucosal surfaces, lowering the risk for viral and bacterial infections.12 In vivo models show that 2’FL protects against infections with Campylobacter jejuni,15,16 while LNnT reduces the number of Streptococcus pneumonia cells in the lungs.17
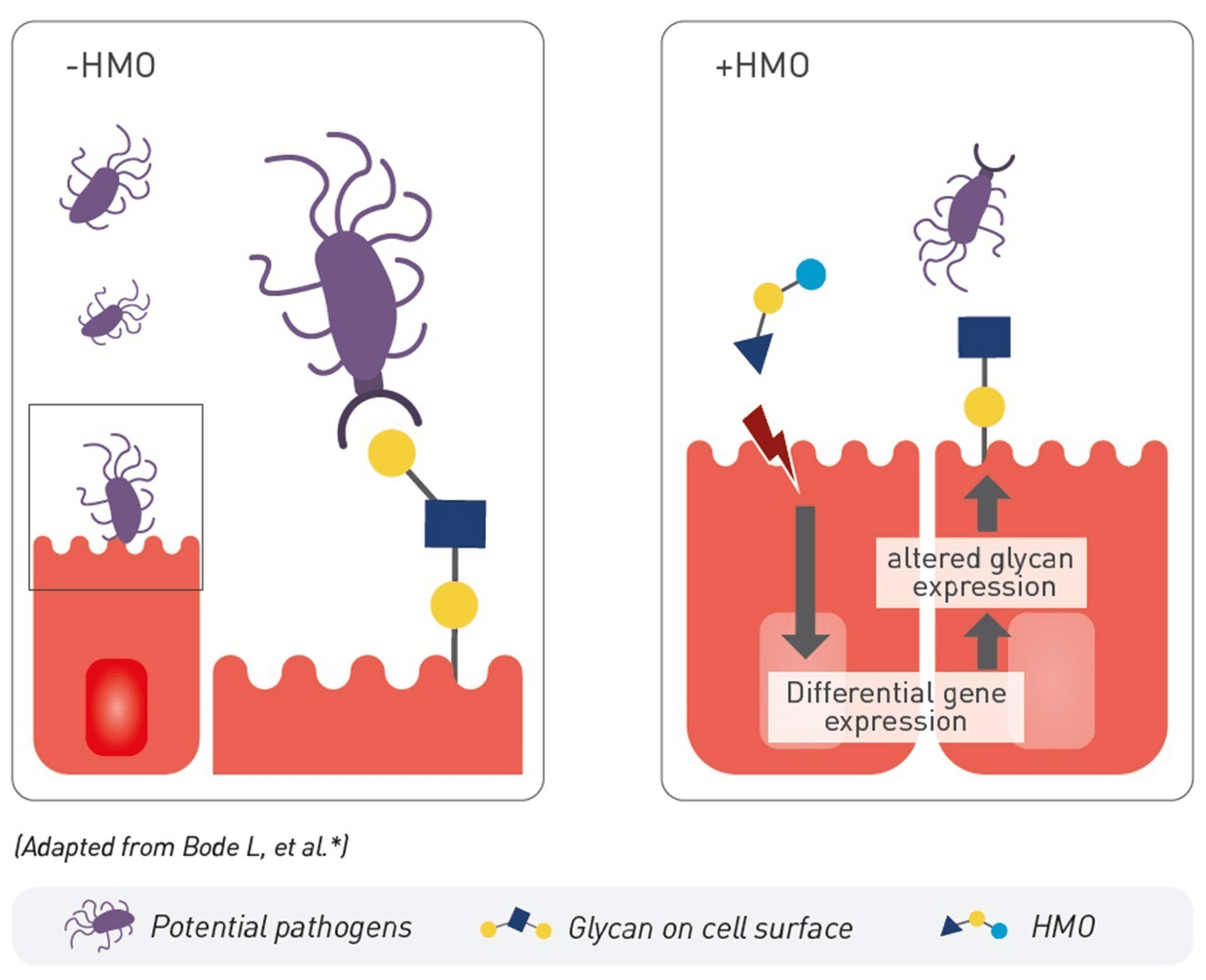
3. SUPPORT OF GUT BARRIER FUNCTION
HMO, including 2’FL and LNnT, strengthen the gut barrier by promoting intestinal cell proliferation and maturation.11 HMO modulate their gene expression, notably tight junction protein and glycocalyx component expression, enhancing the gut barrier function.12
4. IMMUNE SYSTEM MATURATION
HMO act at the systemic level to modulate interactions between immune cells and help maintain a balanced inflammatory response.12,18 HMO, like 2’FL, have anti-inflammatory effects (linked to Th2 activity).12
Structure specific benefits
Until recently, HMO structurally identical to those found in breast milk, but not sourced from breast milk, were not commercially available. The search for alternatives has led to supplementing formula with simple oligosaccharides that could be more easily synthesized but were not found in breast milk, like GOS (galactooligosaccharides) and FOS (fructo-oligosaccharides).19 However, the unique and complex structures of HMO are integral to their role.19 They share structural similarities with mucosal glycans at the host-microbe interface, which supports their roles in orchestrating the host-microbial interactions via different mechanisms.19 Subsequently, simple oligosaccharides, like GOS and FOS, may not replicate the benefits of HMO.19
Food allergies and the immune system
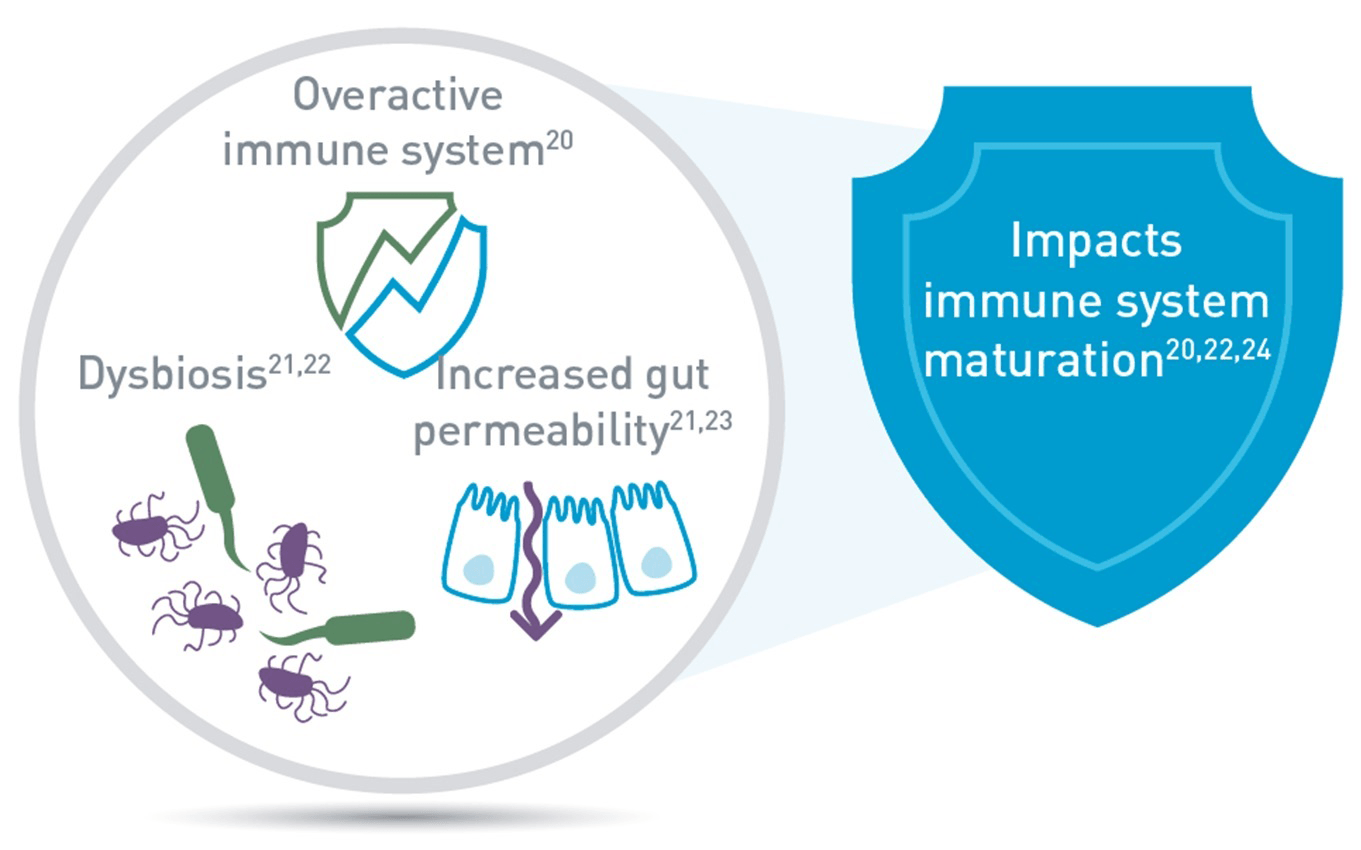
CMPA is an immune-mediated disease.20 Infants with CMPA have been shown to have an increased gut permeability and an imbalanced microbiota (dysbiosis)20-23 which may affect the maturation of their immune system.3,4,24 Studies have shown:
- A four-fold increase in the risk of respiratory infections in children aged 1-2 years old with food allergies,25 whilst the risk of recurrent otitis media is doubled during childhood.26,27
- The risk of developing atopic manifestations, such as asthma, atopic eczema, and respiratory allergies is 3 or 4 times higher, at 10 years of age in children who had CMPA in infancy.26
BRINGING THE BEFENITS OF HMO TO NON-BREASTFED INFANTS WITH CMPA
Breastmilk is the gold standard source of nutrition for all infants.5 When an infant with CMPA cannot be breastfed for any reason, specialty hypoallergenic formulas are required.28
For decades, Nestlé has studied HMO. This has led to 5 clinical trials in both healthy infants and infants with CMPA, investigating the benefits of supplementing standard and specialty hypoallergenic formula with 2’FL and LNnT, not sourced from, but structurally identical to these two HMO found in breast milk.29-34
HMO production has recently become technically feasible, and our range of specialty formulas, Althéra® HMO, Alfaré® HMO and Alfamino® HMO, are the first to be supplemented with 2’FL and LNnT. They are proven hypoallergenic and effectively relieve infants from the symptoms of CMPA, whilst the addition of 2’FL and LNnT has been shown to reduce the risk of infections, reduce associated medication use and promote a microbiota closer to healthy breastfed infants.29,32,34
IMPORTANT NOTICE:
Mothers should be encouraged to continue breastfeeding even when their infants have cow’s milk protein allergy. This usually requires qualified dietary counseling to completely exclude all sources of cow’s milk protein from the mothers’ diet. If a decision to use a special formula intended for infants is taken, it is important to give instructions on correct preparation methods, emphasizing that unboiled water, unsterilized bottles or incorrect dilution can all lead to illness. Formula for special medical purposes intended for infants must be used under medical supervision.
REFERENCES:
- Robertson RC, et al. Trends Microbiol. 2019;27(2):131-47
- Agosti M, et al. Pediatr Med Chir. 2017;39(2):157
- Dzidic M, et al. Med Sci (Basel). 2018;6(3):56
- Chin AM, et al. Semin Cell Dev Biol. 2017;66:81-93
- Newton ER. Clin Obstet Gynecol. 2004;47(3):632-42
- Ayechu-Muruzabal V, et al. Front Pediatr. 2018;6:239
- Arrieta MC, et al. Front Immunol. 2014;5:427
- Duijts E, et al. Pediatrics. 2010;126(1):e18-25
- Oddy WH. Ann Nutr Metab. 2017;70(Suppl 2):26-36
- Scholtens S, et al. Thorax. 2009;64(7):604-9
- Donovan SM and Comstock SS. Ann Nutr Metab 2016;69(Suppl 2):42-51
- Walsh C, et al. J Funct Foods. 2020;72:104074
- Azad MB, et al. J Nutr. 2018;148(11):1733-42
- van den Abbeele, et al. J Funct Foods. 2019;61:103484
- Ruiz-Palacios GM, et al. J Biol Chem. 2003;278(16):14112-20
- Morrow AL, et al. J Pediatr. 2004;145(3):297-303
- Idänpään-Heikkilä I, et al. J Infect Dis. 1997;176(3):704-12
- He Y, et al. Mucosal Immunol. 2014;7(6):1326-39
- Bode L and Jantscher-Krenn E. Adv Nutr.2012;3(3):383S–391S
- Crittenden RG and Bennett LE. J Am Coll Nutr. 2005;24(6):582S–591S
- Thompson-Chagoyan OC, et al. Allergy Int Arch Allergy Immunol. 2011;156(3):325–32
- Azad MB, et al. Clin Exp Allergy. 2015;45(3):632-43
- Jalonen T. J allergy Clin Immunol. 1991;88(5):737-42
- Tanaka M and Nakayama J. Allergol Int. 2017;66(4):515-22
- Woicka-Kolejwa K, et al. Postepy Dermatol Alergol. 2016;33(2):109-13
- Juntti H, et al. Acta Otolaryngol.1999;119(8):867-73
- Tikkanen S, et al. Acta Paediatr. 2000;89(10):1174-80
- Koletzko S, et al. J Pediatr Gastroenterol Nutr. 2012;55(2):221-29
- Puccio G, et al. J. Pediatr Gastroenterol Nutr. 2017;64(4):624-31
- Nowak-Wegrzyn A, et al. Nutrients. 2019;11(7):1447
- Román E, et al. Nutr Hosp. 2020;10.20960/nh.03084
- Pedersen H, et al. Congress Poster: the FAAM-EUROBAT Digital 2020 meeting.
- Nestlé Health Science, data on file. PLATYPUS study
- Vandenplas Y, et al. Presentation at EAACI Digital Congress, June 2020
* Bode L. Glycobiology 2012;22(9):1147–62
