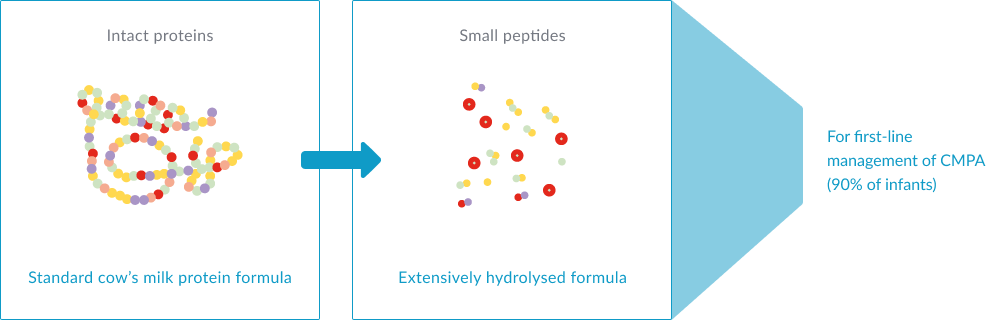
What is an extensively hydrolysed formula?
In most cases where breastfeeding is not an option for infants with CMPA, guidelines recommend an extensively hydrolysed formula (eHF) as the appropriate first-line dietary management, with the ultimate goal being to ensure normal growth and symptom relief. These guidelines also state that an eHF should be well-tolerated by at least 90% of infants with CMPA (with a 95% confidence interval).
In eHFs, allergy-triggering proteins in cow’s milk are broken down into small peptides by hydrolysis, amongst other processes such as ultrafiltration, making them less allergenic than whole milk infant formulas. eHFs are more palatable than amino acid-based formulas (AAFs), which are generally reserved for severe CMPA, which might help improve formula intake and long-term growth.2,5,6 Some eHFs, like Althéra® HMO, also contain purified lactose, which is beneficial for calcium absorption and also helps to positively modulate the gut microbiota. 1-2,7-8
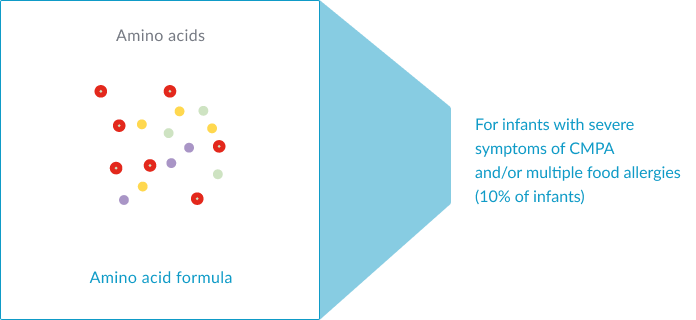
Amino acid-based formulas (AAFs): prepared from free amino acids. AAFs, such as Alfamino® HMO, are recommended in infants with severe symptoms of CMPA and/or multiple food allergies.1-3,14
Once an infant has eliminated cow’s milk protein from their diet with an appropriate specialty formula, CMPA symptoms typically resolve between 1-4 weeks, and sometimes sooner.2 To confirm or exclude the diagnosis, a controlled oral food challenge under medical supervision is often required.
Are all eHFs equally effective?
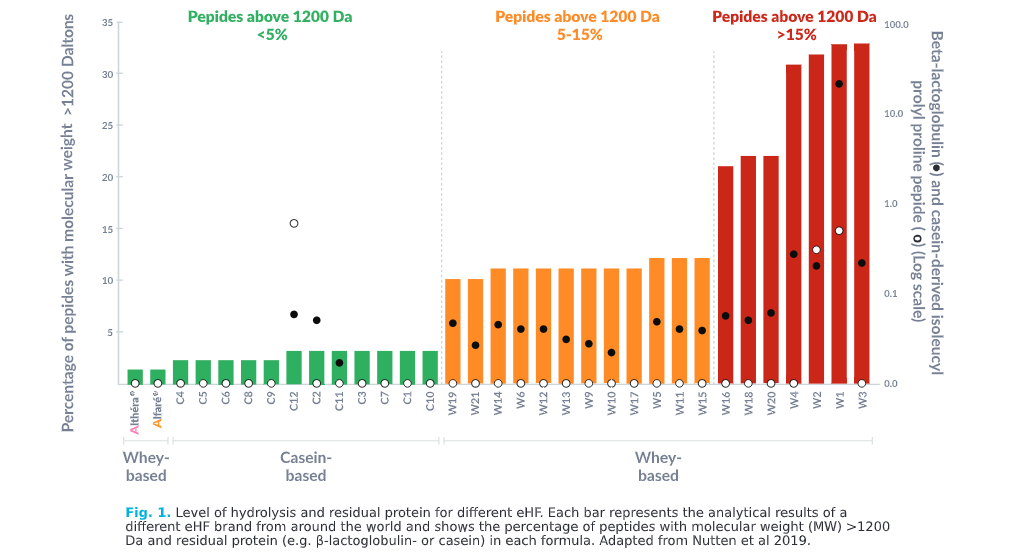
There are no clear guidelines for the degree of hydrolysis or size of peptides for extensively hydrolysed formulas for the management of CMPA. There is also no standardised protocol to test for residual allergenicity or hypoallergenicity.
As a result, despite having the same objective, no eHF looks alike, and there are many available with a large variability between them. Furthermore, not all products have been tested appropriately or to the same high standards, and some eHFs have been reported as poorly tolerated or to have residual peptides that could be sufficient to provoke an allergic reaction. 9-10
51%
For example, in the Dutch cohort of the EuroPrevall study, one eHF brand with 5-15% peptides above 1200 Da (Fig 1. orange) did not achieve adequate symptom control in 51% of infants with CMPA, resulting in the reintroduction of an AAF.9
How peptide size is linked to hypoallergenicity
A recent analysis of a large number of eHFs demonstrated notable variations in:11



Larger pepide size and residual protein may impact eHF clinical efficacy and risk of allergic reacions

Innovation that’s backed by 150 years of expertise
At Nestlé, we drew on our 150 years of expertise in infant nutrition to develop hypoallergenic extensively hydrolysed formulas that are safe, effective and support healthy growth and development in infants with CMPA.
We developed an innovative, rigorous and patented hydrolysis process to ensure that cow’s milk proteins are broken down to a level that is safe for infants with CMPA.
Our two eHFs, Althéra® HMO and Alfaré® HMO, are the most extensively hydrolysed and contain the lowest percentage of peptides above 1200 Daltons, and they have very low residual allergen content11
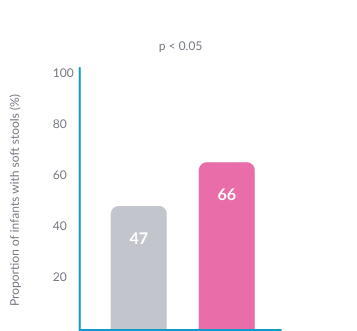
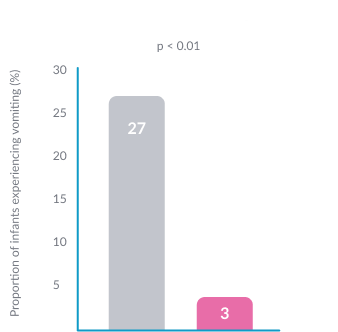
This is thought to contribute to Althéra® HMO being the only eHF to demonstrate similar efficacy and safety as an amino-acid based formula, which are generally only reserved for those with severe CMPA.15 It is also significantly better tolerated than an AAF.15
Higher frequency of soft stools15
Lower frequency of vomiting15
Our range of immune-nurturing formulas for cow’s milk protein allergy
*Structurally identical Human Milk Oligosaccharides (HMO) are not sourced from human milk
REFERENCES
- Koletzko S, et al. Diagnostic Approach and Management of Cow’s-Milk Protein Allergy in Infants and Children: ESPGHAN GI Committee Practical Guidelines. J Pediatr Gastroenterol Nutr 2012;55(2):221-229.
- Prescott SL, et al. A global survey on changing patterns of food allergy burden in children. World Allergy Organ J. 2013;6(1) :21
- Cekola P, et al. Clinical use and safety of an amino acid-based infant formula in a real world setting. Abstract presented at NASPGHAN. Chicago, IL, USA, October 2019.
- Høst A. Cow’s milk protein allergy and intolerance in infancy. Some clinical, epidemiological and immunological aspects. Pediatr Allergy Immunol. 1994;5:1-36.
- Jalonen T. Identical intestinal permeability changes in children with different clinical manifestations of cow’s milk allergy. J allergy Clin Immunol 1991;88(5):737-742.
- Crittenden RG and Bennett LE. Cow’s Milk Allergy: A Complex Disorder. J Am Coll Nutr 2005;24(6suppl):582S–591S.
- Maslin K, et al. Palatability of hypoallergenic formulas for cow’s milk allergy and healthcare professional recommendation. Pediatric Allergy and Immunol. 2018; 29:857-862.
- Francavilla R, et al. Effect of lactose on gut microbiota and metabolome of infants with cow’s milk allergy. Pediatr Allergy Immunol. 2012;23:420-427.
- Abrams SA, et al. Calcium and zinc absorption from lactose-containing and lactose-free infant formulas. Am J Clin Nutr. 2002;76(2):442-6.
- Petrus NCM, et al. Remaining symptoms in half the children treated for milk allergy. Eur J Pediatr. 2015; 174:759-765.
- Chauveau, A, et al. Immediate hypersensitivity to extensively hydrolysed formulas: An important reminder. Pediatric Allergy and Immunology. 2016;27(5):541-543.
- Nutten S, et al. Peptide size profile and residual immunogenic milk protein or peptide content in extensively hydrolysed infant formulas. Allergy. 2019
- Nowak-Wegrzyn A, et al. Confirmed Hypoallergenicity of a Novel Whey-Based Extensively Hydrolyzed Infant Formula Containing Two Human Milk Oligosaccharides. Nutrients. 2019;11(7):E1447.
- Nowak-Wegrzyn A, et al. Hypoallergenicity of a whey-based, extensively hydrolysed infant formula prepared with nonporcine enzymes. Allergy. 2019;74(8):1582-1584.
- Niggemann, B, et al. Safety and efficacy of a new extensively hydrolysed formula for infants with cow’s milk protein allergy. Pediatr Allergy Immunol. 2008. 19(4):348-54.
- Vandenplas Y, et al. A workshop report on the development of the Cow’s Milk-related Symptom Score awareness tool for young children. Acta Paediatr. 2015;104(4):334-339.
- Vandenplas Y, et al. Pooled analysis of the Cow’s Milk-related Symptom Score (CoMiSSTM) as a predictor for cow’s milk related symptoms. Pediatr Gastroenterol Hepatol Nutr. 2017;20(1):22-26.
- Vandenplas Y, et al. The Cow Milk Symptom Score (CoMiSSTM) in presumed healthy infants. PLoS One. 2018;13(7):e0200603.
- Nestlé Health Science, data on file. CINNAMON study.

