Advancing the management of cow’s milk protein allergy
Accumulating evidence highlights that nutrition in early life may influence the risk of obesity in later life. Breastfeeding is the gold standard in infant nutrition and has been identified as a protective factor against obesity compared to infant formula, attributable in part to the optimal and lower protein content of human milk. Nestlé has pioneered a unique process to reduce the protein content in infant formula. Recently, this process has been applied to a whey based extensively hydrolyzed formula (eHF), Althéra® HMO, indicated when breastfeeding is not possible, as first-line management for infants with cow’s milk protein allergy (CMPA).
Breastfeeding can reduce the risk of obesity
The global prevalence of overweight and obese children has risen 47% between 1980 and 2013.1 Nutritional factors during early life, particularly protein intake, have been shown to modulate later obesity risk, via a phenomenon called metabolic programming.2,3
Breast milk is undoubtedly the gold standard source of nutrition for all infants,4 including those with CMPA.5 Its nutrient profile is dynamic, evolving throughout lactation, in response to the changing requirements of an infant. The protein level in breastmilk ranges from ~1.4-1.7g/100kcal in the first 6 months, down to ~1.3-1.5g/100kcal from 6-24 months post-partum.6
Conversely, infant formulas contain more protein at ~1.8–2.9g/100kcal, in order to supply adequate quantities of indispensable and conditionally indispensable amino acids that are available in breastmilk.9
Compared to infant formulas, breastfeeding has a protective effect against obesity, in part due to its optimal and lower protein content.2,7
The Early Protein Hypothesis
Accumulating evidence supports the ‘Early Protein Hypothesis’.2,8 It has been observed that infants fed higher protein formula have more rapid growth in the first 2 years of life (Fig. 1)9 and that there is an increased risk of obesity until at least 6 years of age (Fig. 2).10 Infants fed lower protein formula showed growth patterns which were more similar to those of breast fed infants.8
Figure 1: Significantly different from the lower protein group (ANOVA adjusted for baseline value): * p<0.05, ** p<0.01, *** p<0.001. Adapted from Koletzko B, et al9

Risk of being obese at 6 years of age with higher-protein formula:
- x2.84 vs. breastfed infants (p=0.063)
- x2.43 vs. low protein formula-fed infants (p=0.024)
Figure 2: The risk of becoming obese. Adapted from Weber M, et al10
The potential mechanism of the Early Protein Hypothesis
High protein intake in early life increases circulating concentrations of plasma essential amino acids (in excess of requirements) and insulin-like growth factor-1 (IGF-1) which results in an increase in fat mass.2,11,12 This early life experience is thought to result in “metabolic programming” which can influence the regulation of body weight throughout life.
Protein requirements of infants with CMPA
The management of CMPA has until recently largely focused on short-term health outcomes, such as symptom relief, while many long-term health outcomes associated with infant nutrition, such as optimisation of the protein level, have not been addressed.
The protein requirements of infants with CMPA are normally the same as healthy infants, unless there are severe gut pathologies related to CMPA, causing malabsorption and faltering growth. If breast feeding is not possible, amino acid-based formulas, which have high levels of protein, are generally indicated in these instances.5,13,14 However, if there is absence of gut pathology related to CMPA, a whey-based eHF containing lactose will provide effective first line support.5,13, 14
Nestlé Health Science pioneers a lower protein eHF for CMPA
Inspired by breast milk, Nestlé were pioneers in the development of regular infant formula with reduced levels of high quality whey protein, which still continue to provide all indispensable and conditionally indispensable amino acids.11,15 On this basis, Nestlé Health Science have pioneered a whey-based eHF for the first-line management of CMPA (Althéra® HMO), which has the lowest protein level of all available eHF:
| Althéra® HMO (new formulation) |
Althéra® (formulation without HMO) |
Competitor casein-based eHF16 |
|---|---|---|
| 2.20g/100kcal | 2.50g/100kcal | 2.79g/100kcal |
| 14% higher vs. Althéra® HMO | 27% higher vs. Althéra® HMO |
The CINNAMON trial
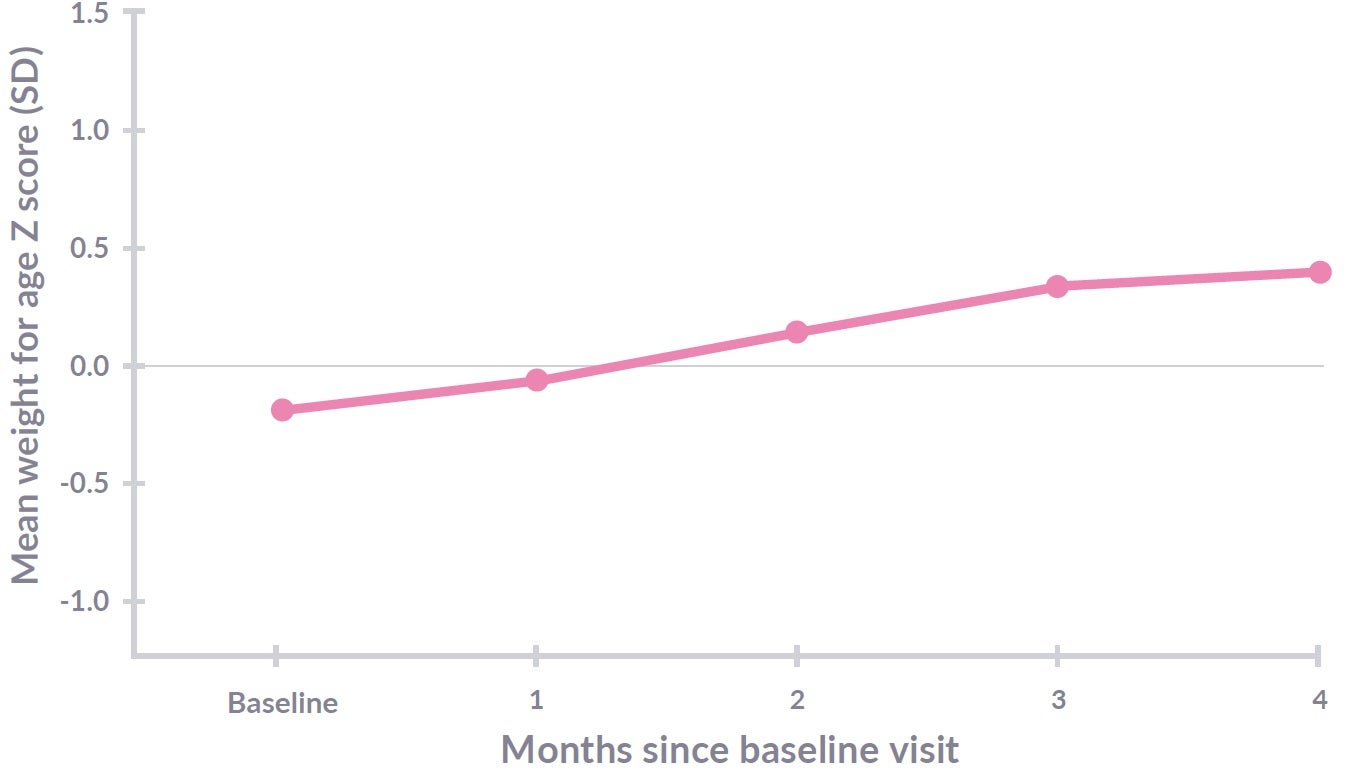
Figure 3: Althéra® with 2’FL and LNnT (protein 2.20g/100kcal) n=94. Weight gain from visit 0 (baseline) to visit 4 (4 months; Primary endpoint). Weight gain to visit 6 - data pending (12 months of age; secondary endpoint). Adapted from Vandenplas Y, et al17
In the CINNAMON trial, Althéra® HMO, with its lower protein content, was shown to be safe, to provide effective symptom relief, reduce infections and positively modulate the gut microbiota of infants with CMPA.17-19 The primary outcome, to ensure normal growth (weight, length and head circumference) for 4 months from baseline was achieved and was also observed up to 12 months of age.17,18
ADVANCING THE MANAGEMENT OF CMPA IN THE SHORT AND LONG TERM
Childhood obesity is a growing challenge which impacts quality of life and healthcare systems.1
Breast feeding provides infants the best start in life, including reducing the future risk of obesity. In order to ensure that non-breastfed infants with CMPA can experience benefits inspired by breast milk, Nestlé Health Science have pioneered a whey-based eHF for the first-line management of CMPA with lower levels of protein.
The CINNAMON trial demonstrated that the whey-based eHF (Althéra® HMO) was associated not only with normal growth, but also effective symptom relief, a reduction in respiratory tract infections and positive gut microbiome modulation.17-19
IMPORTANT NOTICE:
Mothers should be encouraged to continue breastfeeding even when their infants have cow’s milk protein allergy. This usually requires qualified dietary counseling to completely exclude all sources of cow’s milk protein from the mothers’ diet. If a decision to use a special formula intended for infants is taken, it is important to give instructions on correct preparation methods, emphasizing that unboiled water, unsterilized bottles or incorrect dilution can all lead to illness. Formula for special medical purposes intended for infants must be used under medical supervision.
REFERENCES:
- Ng et al. Lancet 2014;388:766-81
- Koletzko B, et al. Am J Clin Nutr 2009;89(suppl):1502S–8S
- Haschke F, et al. NNI Workshop Series 2016;85:pp101-109
- Newton ER. Clin Obstet Gynecol 2004;47(3):632-42
- Koletzko S, et al. J Pediatr Gastroenterol Nutr 2012;55(2):221-29
- Lonnerdal B, et al. J Nutr Biochem 2017;41:1-11
- Koletzko B, et al. Adv Exp Med Biol 2009;646:15-29
- Koletzko B, et al. Semin Perinatol 2019;43(7):151153
- Koletzko B, et al. Am J Clin Nutr 2009;89:1836–45
- Weber M, et al. Am J Clin Nutr 2014;99:1041–51
- Alexander DD, et al. Am J Clin Nutr 2016;104:1083–92
- Desai M, et al. Curr Diab Rep 2013;13:27-33
- Muraro A, et al. EMJ Allergy Immunol 2017;2(1):46-51
- Baker SS, et al. Pediatrics 2000;106;346-9
- US patent 6787158 B1. Sept 7, 2014
- https://www.nutramigen.co.uk/products/mild-to-moderate-cows-milk-allergy/nutramigen-1-with-lggreg/ [Last accessed 02 February 2021]
- Vandenplas Y, et al. Abstract presented at PAAM. Florence, Italy, October 19, 2019
- Vandenplas Y, et al. Abstract presented at EAACI Digital Congress, June 2020
- Pedersen H, et al. Congress Poster: the FAAM-EUROBAT Digital 2020 meeting
