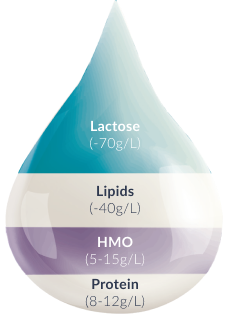
Human Milk Oligosaccharides (HMO)
COW’S MILK PROTEIN ALLERGY MAY AFFECT IMMUNE SYSTEM MATURATION
The first years of life are crucial in setting the foundations for future health, but it is also when infants are most vulnerable1 and more susceptible to infections,2,3 especially those who are formula-fed.4,5
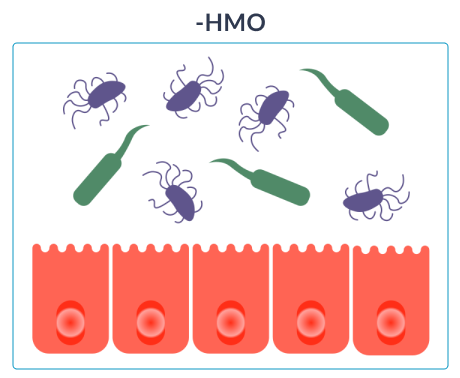
Cow’s milk protein allergy (CMPA) is associated with an imbalanced gut microbiota (dysbiosis), increased gut permeability and an overactive immune system. Infants with CMPA therefore face an even greater risk of infections and future allergies.24,28,33,34.
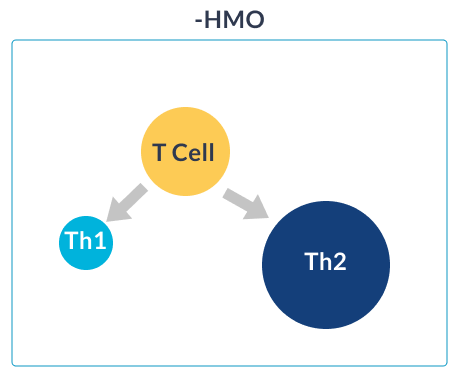
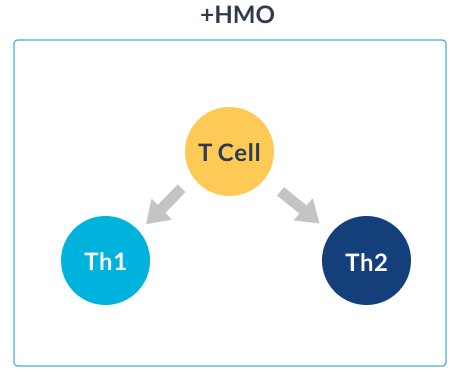
HMO SUPPORT IMMUNE SYSTEM MATURATION
The gold standard in infant nutrition, breast milk, has been shown to reduce the incidence of infectious diseases and allergies in infants.5,9-15 This is attributed to diverse bioactive components present in breast milk, such as human milk oligosaccharides (HMO).16 4,5
Several clinical studies have reported on the protective effect of HMO in healthy breastfed infants, including a reduction of the:
Gastroenteris and diarrhea11-14
Respiratory tract infections13,14
Atopic manifestations, including CMPA14-16
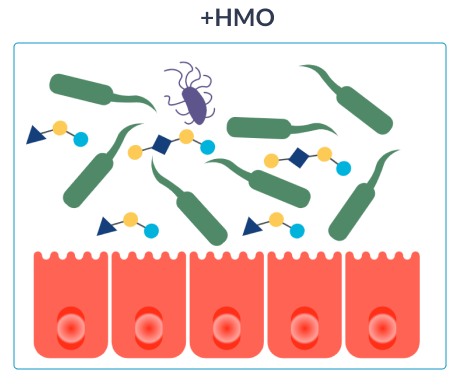
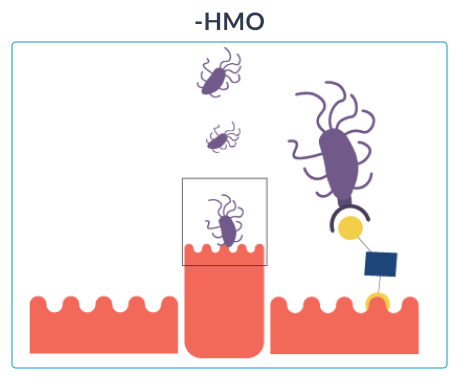
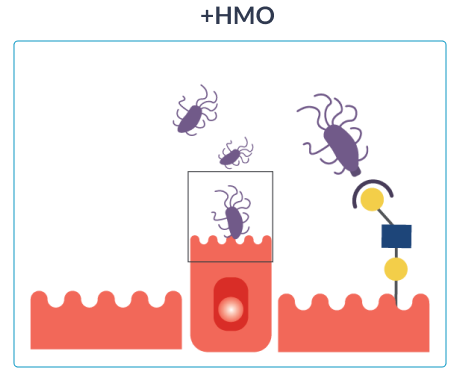
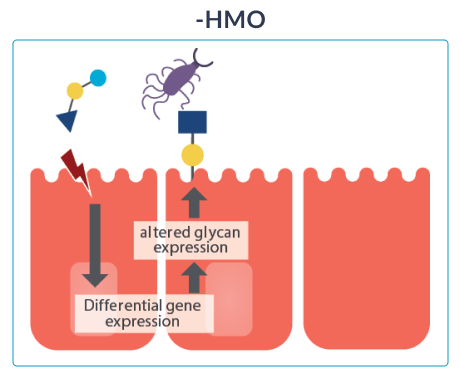
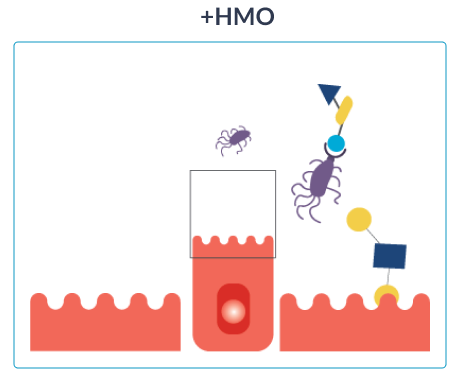
HOW HMO NURTURE INFANTS IMMUNE SYSTEMS
HMO ADVANCE THE MANAGEMENT OF CMPA
Althéra ® HMO, Alfaré ® HMO, Alfamino ® HMO and Alfamino ® Junior HMO are the only range of specialty formulas with structurally identical 2’FL and LNnT. *
Our specialty formulas with 2’FL and LNnT for CMPA have been proven to be hypoallergenic, well tolerated and to support effective symptom relief and normal growth. 19,20,23 In both healthy infants and infants with CMPA, infant formulas with 2’FL and LNnT have also been proven to promote a microbiota closer to that of healthy breastfed infants and reduce infection and medication use, including: 20,22,24,25
Once ingested, HMO resist the low stomach pH as well as degradation through pancreatic and brush border enzymes in the small intestine. Data from breastfed infants show that approximately 1% of the ingested HMO are absorbed.16 Their effects extend to tissues and organs other than the intestine. Most HMO reach the distal small intestine and colon intact where they are either metabolised by microbes or excreted with the faeces.
2’FL and LNnT are among the 10 most abundant HMO in human breast milk.17,29-31 They are the most researched HMO in preclinical and clinical studies. Infant formulas supplemented with 2’FL and LNnT have been shown to have a positive effect on immunity, support normal growth and be well tolerated.2,16,20-23,32-34 2’FL and LNnT are furthermore approved as safe by the European Food Safety Agency (EFSA).26-27,35-36
GOS (galacto-oligosaccharides) and FOS (fructo-oligosaccharides) are two simple oligosaccharides not found in human milk. The structure of HMO is different from that of GOS and FOS: HMO are more complex which is essential for the immune-nurturing roles of HMO.18
HMO may have an impact on the development of some allergies in children during the first two years of life. First evidence from a clinical study showed that an abundance of 2’ fucosylated HMO, especially 2ʹFL, in human breast milk may reduce the risk of immunoglobulin E (IgE)-associated eczema in breastfed infants with high risk of allergy at the age of 2 years, when born by C-section.14
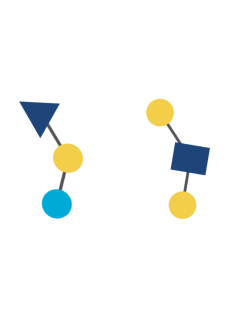
Out of over 200 unique types of HMO that have been identified so far,16 2’fucosyllactose (2’FL) and lacto-N-neotetraose (LNnT) are among the 10 most abundant oligosaccharides in human breast milk (2’FL, LNnT, 6’SL, 3’SL, 3’FL, LNT, LNFP-I, LNFP-II, LNFP-III, DFLNT), which collectively constitute more than 75% of total HMO.17 2’FL and LNnT are also among the most researched HMO.
Due to their highly complex structure, manufacturing structurally identical HMO is technically challenging. Breakthrough advances in biotechnology after almost 30 years of research and development by Nestlé along with their partners, has enabled the commercial production of HMO*, such as 2’Fucosyllactose (2’FL) and lacto-N-neotetraose (LNnT).18,26,27
Multiple studies and trials also had to be first conducted to assess the safety and hypoallergenicity of HMO in the target populations when supplemented into infant and specialty formulas.19,23,28
.
REFERENCES:
-
Nowak-Węgrzyn A, et al. Evaluation of Hypoallergenicity of a New, Amino Acid–Based Formula. Clin Pediatr (Phila) 2015;54:264–272.
-
Nowak-Wegrzyn A, et al. Confirmed Hypoallergenicity of a Novel Whey-Based Extensively Hydrolyzed Infant Formula Containing Two Human Milk Oligosaccharides. Nutrients 2019;11(7):E1447.
-
Cekola P, et al. Clinical use and safety of an amino acid-based infant formula in a real world setting. Abstract presented at NASPGHAN. Chicago, IL, USA, October 2019.
-
Koletzko S, et al. Diagnostic Approach and Management of Cow’s-Milk Protein Allergy in Infants and Children: ESPGHAN GI Committee Practical Guidelines. J Pediatr Gastroenterol Nutr 2012;55(2):221-229.
-
Luyt D, et al. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin Exp Allergy 2014;44(5):642-672.
-
Muraro A, et al. EAACI Food Allergy and Anaphylaxis Guidelines: diagnosis and management of food allergy. Allergy 2014;69(8):1008–1025.
-
Bode L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012;22(9):1147-1162.
-
Donovan SM and Comstock SS. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann Nutr Metab 2016;69(suppl 2):42-51.
-
Nestlé Health Science, data on file. CINNAMON study.
-
Vandenplas Y, et al. Growth, tolerance and safety of an extensively hydrolysed formula containing two human milk oligosaccharides in infants with cow’s milk protein allergy. Abstract presented at PAAM. Florence, Italy, October 19, 2019.
-
Puccio G, et al. Effects of Infant Formula With Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial. J Pediatr Gastroenterol Nutr 2017;64(4):624-631.
-
Heine RG, et al. Lactose intolerance and gastrointestinal cow’s milk allergy in infants and children – common misconceptions revisited. World Allergy Organ J 2017;10(1):41.
-
Delplanque B, et al. Lipid Quality in Infant Nutrition: Current Knowledge and Future Opportunities. J Pediatr Gastroenterol Nutr 2015;61(1):8-17.
-
Bach AC, et al. Medium-chain triglycerides : an update. Am J Clin Nutr 1982;36(5):950-962.
-
Kennedy K, et al. Double-blind, randomized trial of a synthetic triacylglycerol in formula-fed term infants: effects on stool biochemistry, stool characteristics, and bone mineralization. Am J Clin Nutr 1999;70(5):920-927.
-
Koletzko B. Human milk lipids. Ann Nutr Metab 2016;69(suppl 2):28-40.
-
Mazzocchi A, et al. The Role of Lipids in Human Milk and Infant Formulae. Nutrients 2018;10(5):E567.
-
American Academy of Pediatrics, Committee on Nutrition. Pediatrics 1976;57:278-285.
-
Nestlé Health Science, data on file. Alfamino® product composition.
-
Corkins M, et al. Assessment of Growth of Infants Fed an Amino Acid-Based Formula. Clin Med Insights Pediatr 2016;10:3–9.
-
Vandenplas Y, et al. Growth in infants with cow’s milk protein allergy fed an amino acid-based formula. Abstract N-O-013 presented at ESPGHAN, 2019. J Pediatr Gastroenterol Nutr 2019;68(Suppl 1).
-
Nestlé Health Science, data on file. Alfamino® versus Neocate® competitive benchmarking test.
-
Host A and Halken S. Cow's milk allergy: where have we come from and where are we going? Endocr Metab Immune Disord Drug Targets 2014;14(1):2-8.
-
Azad MB, et al. Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy 2015;45(3):632-643.
-
West CE, et al. The gut microbiota and its role in the development of allergic disease: a wider perspective Clin Exp Allergy 2015;45(1):43–53.
-
Thompson-Chagoyan OC, et al. Faecal Microbiota and Short-Chain Fatty Acid Levels in Faeces from Infants with Cow‘s Milk Protein Allergy Int Arch Allergy Immunol 2011;156(3):325–332.
-
Chin AM, et al. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin Cell Dev Biol 2017;66:81-93.
-
Tanaka M and Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life Allergol Int 2017;66(4):515-522.
-
Newburg DS and Walker WA. Protection of the Neonate by the Innate Immune System of Developing Gut and of Human Milk. Pediatr Res 2007;61(1):2-8.
-
Woicka-Kolejwa K, et al. Food allergy is associated with recurrent respiratory tract infections during childhood. Postepy Dermatol Alergol 2016;33(2):109-113.
-
Juntti H, et al. Cow’s Milk Allergy is Associated with Recurrent Otitis Media During Childhood. Acta Otolaryngol 1999;119(8):867-873.
-
Tikkanen S, et al. Status of children with cow’s milk allergy in infancy by 10 years of Age. Acta Paediatr 2000; 89(10):1174-1180.
-
Jalonen T. Identical intestinal permeability changes in children with different clinical manifestations of cow’s milk allergy. J allergy Clin Immunol 1991;88(5):737-742.
-
Crittenden RG and Bennett LE. Cow’s Milk Allergy: A Complex Disorder. J Am Coll Nutr 2005;24(6suppl):582S–591S.
IMPORTANT NOTICE:
Mothers should be encouraged to continue breastfeeding even when their infants have cow’s milk protein allergy. This usually requires qualified dietary counseling to completely exclude all sources of cow’s milk protein from the mothers’ diet. If a decision to use a special formula intended for infants is taken, it is important to give instructions on correct preparation methods, emphasizing that unboiled water, unsterilized bottles or incorrect dilution can all lead to illness. Formula for special medical purposes intended for infants must be used under medical supervision.