ALTHÉRA® HMO:
ADVANCING THE MANAGEMENT OF COW’S MILK PROTEIN ALLERGY
- Extensively hydrolysed, hypoallergenic, whey-based formula (eHF) for effective first-line relief from symptoms of cow’s milk protein allergy (CMPA)†1-6
- Contains lactose, 2’FL and LNnT†, to nurture infants’ developing immune systems6-13
- Supports normal growth and development1,2,6,7,14-17
- Preferred taste over other eHF to help support long-term acceptance18-20
- Nutritionally complete. Suitable as a sole source of nutrition from birth or supplementary feeding from 6 months of age
EFFECTIVELY RELIEVES SYMPTOMS AND SUPPORTS HEALTHY GROWTH
Infants with CMPA may face an immediate challenge from gastrointestinal, skin and/or respiratory symptoms.21-24 Althéra® HMO is proven to be hypoallergenic, with a very low allergenic profile, as a result of Nestlé Health Science’s state-of-the-art hydrolysis process.1-5 Althéra® HMO has been shown to effectively relieve infants from the symptoms of CMPA 1-4,6,7 as well as support normal growth.1,2,6,7,15-17

Skin symptoms
(e.g. eczema, urticaria…)

Gastrointestinal symptoms
(e.g. vomiting, colic, diarrhoea…)
Respiratory symptoms
(e.g. wheezing…)

General symptoms
(e.g. inconsolable crying, failure to thrive…)
Immune-nurturing benefits
CMPA is an immune-mediated disease. It is associated with gut microbiota dysbiosis, which impacts immune system maturation and leaves infants at an increased risk of infections and future allergies.8,25-33 Althéra® HMO contains lactose, as well as 2’-fucosyllactose (2’FL) and lacto-N-neotetraose (LNnT). 2’FL and LNnT are structurally identical to two significant human milk oligosaccharides (HMO).†8-13,34,35

Lactose and HMO support the growth of beneficial bacteria in the gut

HMO strengthen the gut barrier by promoting intestinal cell maturation
HMO eliminate pathogens through a decoy effect

HMO guide the maturation of the immune system, leading to a more balanced Th1/Th2 response
REDUCING INFECTIONS AND MEDICATION USE
Infants fed formula with 2’FL and LNnT† had fewer upper and lower respiratory tract infections6,7,13
Infants fed formula with 2’FL and LNnT† had lower antibiotic and antipyretic use 6,7,13
MEET Edward
Feeding:
Exclusively formula-fed
Symptoms:
Eczema, diarrhoea, inconsolable crying
Diagnosis:
Suspected CMPA
Fictional patient profile for illustrative purpose only
WHAT WOULD BE YOUR MOST IMPORTANT GOAL FOR EDWARD
- SYMPTOM RELIEF
- SUPPORT GROWTH
- SUPPORT IMMUNE HEALTH
For the majority of infants with CMPA who cannot be breastfed, symptom relief can be achieved by eliminating cow’s milk proteins from the infant’s diet with an extensively hydrolysed formula,21-23 like Althéra® HMO.1-7
However, not all extensively hydrolysed formulas are the same. Althéra® HMO has the smallest peptide size (only 1% peptides above 1200 Daltons), resulting in a lower risk of allergic reactions.5
In an observational trial, an extensively hydrolysed formula with 10% peptides above 1200 Daltons did not achieve adequate allergy symptom control in >50% of infants, requiring amino acid formula reintroduction.5,40
Conversely, in a clinical trial, Althéra® was shown to relieve symptoms in 100% of infants with CMPA and is the only extensively hydrolysed formula to demonstrate similar efficacy and safety as an amino acid formula, which are generally reserved for severe symptoms of CMPA. Infants fed Althéra® also had:1
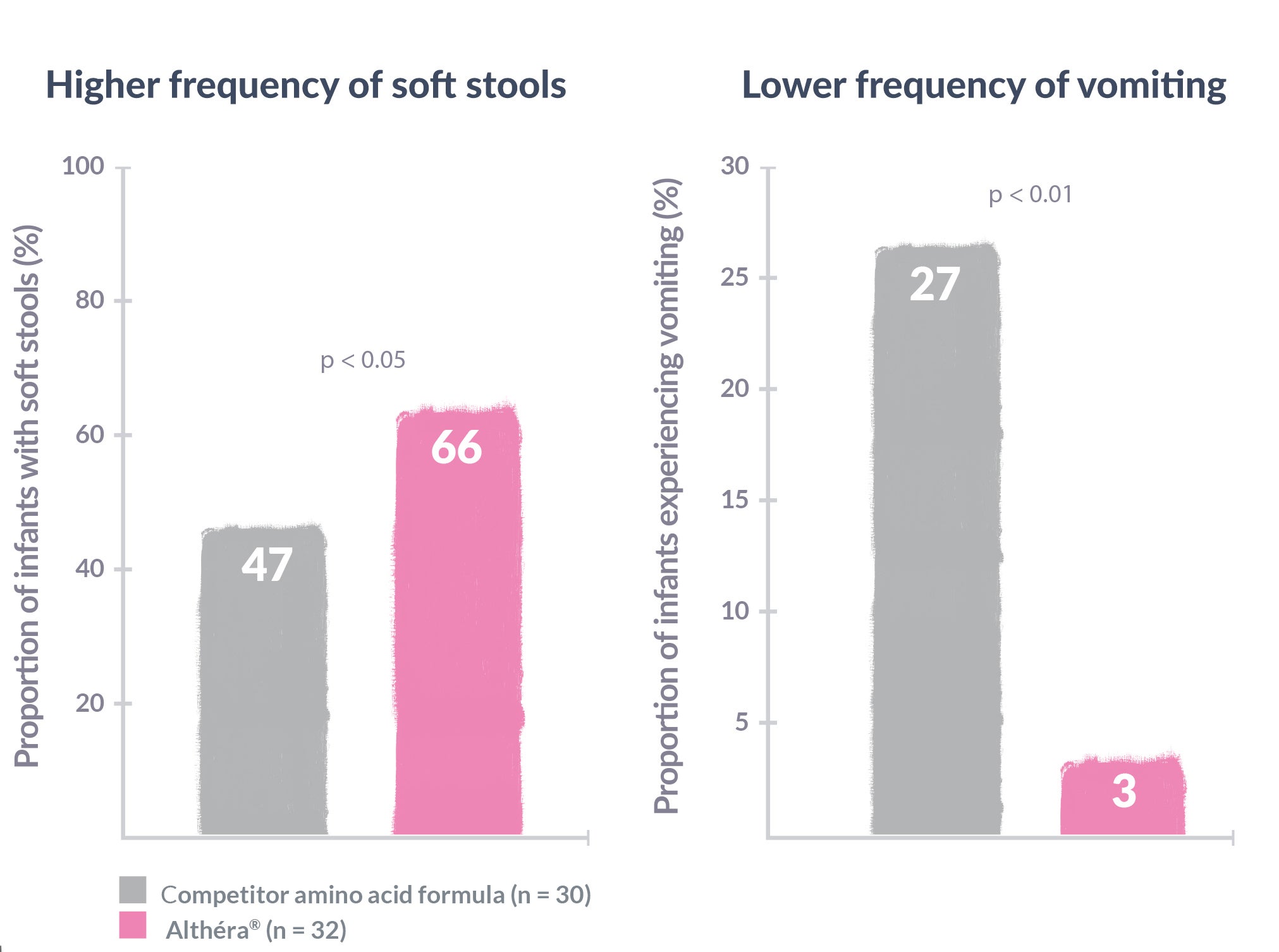
Althéra® HMO is nutritionally complete and suitable as a sole source of nutrition from birth or supplementary feeding from 6 months of age. It has the lowest protein level among all extensively hydrolysed formulas. High protein intake in infancy has been shown to increase the risk of obesity later in life.37,38
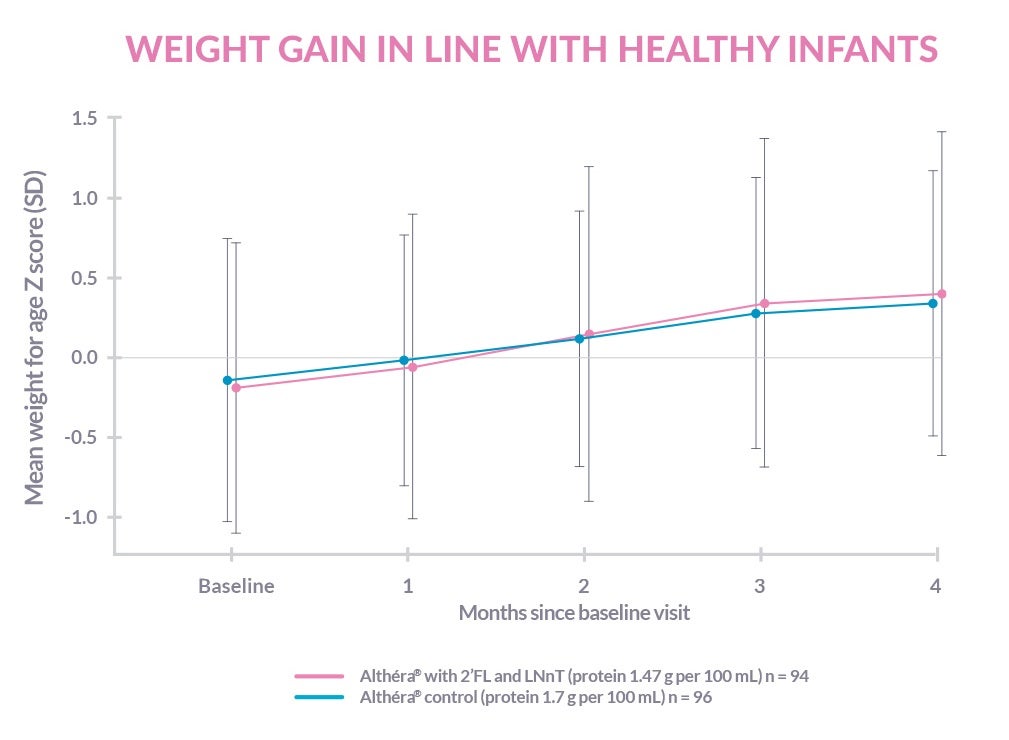
Althéra® HMO is clinically proven to support normal growth and development in infants with CMPA:1,2,6,7
The development of the immune system of infants with CMPA is affected by gut microbiota dysbiosis and increased gut permeability.8,25-27,29
Althéra® HMO is the only extensively hydrolysed formula to contain lactose, 2’FL and LNnT, to support the development of the immune system and reduce the risk of infections and associated medication use:6-8,13,34
| HEALTHY INFANTS8 Mean trial duration: 12 months |
INFANTS WITH CMPA6,7 Mean trial duration: 8.8 months |
|
|---|---|---|
| EAR INFECTIONS | -46%‡ | -71%‡ |
| LOWER RESPIRATORY TRACT INFECTIONS | -55%* | -34%‡ |
| BRONCHITIS | -70%** | |
| GASTROINTESTINAL INFECTIONS | -40%‡ | |
| FREQUENCY OF UPPER RESPIRATORY TRACT INFECTIONS | -42%* | |
| ANTIBIOTIC USE | -53%* | -6%‡ |
| ANTIPYRETIC USE | -56%*** | -11%‡ |
*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.05 at 4 months only; ‡ p = N.S.
FREQUENTLY ASKED QUESTIONS
Our range of immune-nurturing formulas for cow’s milk protein allergy
Our range of nutritional solutions are tailor-made for infants and young children with cow’s milk protein allergy and other food allergies/intolerances. The complete range has been designed to help manage cow’s milk protein allergy earlier, with the right product from the beginning.
†Structurally identical Human Milk Oligosaccharides are not sourced from human milk.
REFERENCES
- Niggemann B, et al. Safety and efficacy of a new extensively hydrolysed formula for infants with cow’s milk protein allergy. Pediatr Allergy Immunol 2008;19(4):348-354.
- Vandenplas Y, et al. Treating cow’s milk protein allergy: a double-blind randomized trial comparing two extensively hydrolysed formulas with probiotics. Acta Paediatr 2013;102(10):990-8.
- Nowak-Wegrzyn A, et al. Confirmed Hypoallergenicity of a Novel Whey-Based Extensively Hydrolyzed Infant Formula Containing Two Human Milk Oligosaccharides. Nutrients 2019;11(7):E1447.
- Nowak-Wegrzyn A, et al. Hypoallergenicity of a whey-based, extensively hydrolysed infant formula prepared with nonporcine enzymes. Allergy 2019;74(8):1582-1584.
- Nutten S, et al. Peptide size profile and residual immunogenic milk protein or peptide content in extensively hydrolysed infant formulas. Allergy 2019. Doi: 10.1111/all.14098.
- Nestlé Health Science, data on file. CINNAMON study.
- Vandenplas Y, et al. Growth, tolerance and safety of an extensively hydrolysed formula containing two human milk oligosaccharides in infants with cow’s milk protein allergy. Abstract presented at PAAM. Florence, Italy, October 19, 2019.
- Francavilla R et al. Effect of lactose on gut microbiota and metabolome of infants with cow’s milk allergy. Pediatr Allergy Immunol 2012;23:420-427.
- Bode L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012;22(9):1147-1162.
- Donovan SM and Comstock SS. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann Nutr Metab 2016;69(suppl 2):42-51.
- Sela DA and Mills DA. Nursing our micriobiota: molecular linkages between bifidobacterial and milk oligosaccharides. Trends Microbiol 2010;18(7):298-307.
- Ayechu-Muruzabal V, et al. Diversity of Human Milk Oligosaccharides and Effects on Early Life Immune Development. Front Pediatr 2018;6:239.
- Puccio G, et al. Effects of Infant Formula With Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial. J Pediatr Gastroenterol Nutr 2017;64(4):624-631.
- Le Huëron-Luron I, et al. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev 2010;23(1):23-3.
- Nutten S. Proteins, Peptides and Amino Acids: Role in Infant Nutrition. Nestle Nutr Inst Workshop Ser. 2016;86:1-10.
- Thakkar SK, et al. Protein Evolution of Human Milk. Nestle Nutr Inst Workshop Ser. 2016;86:77-85.
- Lönnerdal B, et al. Longitudinal evolution of true protein, amino acids and bioactive proteins in breast milk: a developmental perspective. J Nutr Biochem 2017;41:1-11.
- Nestlé Health Science data on file. Althéra® versus Nutramigen® competitive benchmarking test.
- Maslin K, et al. Palatability of hypoallergenic formulas for cow's milk allergy and healthcare professional recommendation. Pediatr Allergy Immunol. 2018;29(8):857-862.
- Miraglia Del Giudice M, et al. Flavor, relative palatability and components of cow’s milk hydrolysed formulas and amino acid-based formula. Ital J Pediatr. 2015;41:42.
- Koletzko S, et al. Diagnostic Approach and Management of Cow’s-Milk Protein Allergy in Infants and Children: ESPGHAN GI Committee Practical Guidelines. J Pediatr Gastroenterol Nutr 2012;55(2):221-229.
- Luyt D, et al. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin Exp Allergy 2014;44(5):642-672.
- Muraro A, et al. EAACI Food Allergy and Anaphylaxis Guidelines: diagnosis and management of food allergy. Allergy 2014;69(8):1008–1025.
- Host A and Halken S. Cow's milk allergy: where have we come from and where are we going? Endocr Metab Immune Disord Drug Targets 2014;14(1):2-8.
- Azad MB, et al. Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy 2015;45(3):632-643.
- West CE, et al. The gut microbiota and its role in the development of allergic disease: a wider perspective Clin Exp Allergy 2015;45(1):43–53.
- Thompson-Chagoyan OC, et al. Faecal Microbiota and Short-Chain Fatty Acid Levels in Faeces from Infants with Cow‘s Milk Protein Allergy Int Arch Allergy Immunol 2011;156(3):325–332.
- Chin AM, et al. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin Cell Dev Biol 2017;66:81-93.
- Tanaka M and Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life Allergol Int 2017;66(4):515-522.
- Newburg DS and Walker WA. Protection of the Neonate by the Innate Immune System of Developing Gut and of Human Milk. Pediatr Res 2007;61(1):2-8.
- Woicka-Kolejwa K, et al. Food allergy is associated with recurrent respiratory tract infections during childhood. Postepy Dermatol Alergol 2016;33(2):109-113.
- Juntti H, et al. Cow’s Milk Allergy is Associated with Recurrent Otitis Media During Childhood. Acta Otolaryngol 1999;119(8):867-873.
- Tikkanen S, et al. Status of children with cow’s milk allergy in infancy by 10 years of Age. Acta Paediatr 2000; 89(10):1174-1180.
- Berger B, et al. Linking Human Milk Oligosaccharides, Infant Fecal Community Types, and Later Risk To Require Antibiotics. mBio 2020;11(2):e03196-19.
- Kunz C, et al. Influence of Gestational Age, Secretor, and Lewis Blood Group Status on the Oligosaccharide Content of Human Milk. Pediatr Gastroenterol Nutr 2017;64(5):789-798.
- Heine RG, et al. Lactose intolerance and gastrointestinal cow’s milk allergy in infants and children – common misconceptions revisited. World Allergy Organ J 2017;10(1):41.
- Rolland-Cachera MF, et al. Nutrient Intakes in Early Life and Risk of Obesity. Int J Environ Res Public Health. 2016;13(6):2-7.
- Haschke F, et al. Early-Life Nutrition, Growth Trajectories, and Long-Term Outcome. Nestle Nutr Inst Workshop Ser. 2019;90:107-120.
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2014. EFSA J 2014;12(7):3760.
- Petrus, N.C.M., Schoemaker, A.A., van Hoek, M.W. et al. Remaining symptoms in half the children treated for milk allergy. Eur J Pediatr 2015;174(6):759-765.
IMPORTANT NOTICE:
Mothers should be encouraged to continue breastfeeding even when their infants have cow’s milk protein allergy. This usually requires qualified dietary counseling to completely exclude all sources of cow’s milk protein from the mothers’ diet. If a decision to use a special formula intended for infants is taken, it is important to give instructions on correct preparation methods, emphasizing that unboiled water, unsterilized bottles or incorrect dilution can all lead to illness. Formula for special medical purposes intended for infants must be used under medical supervision.