ALFARÉ® HMO:
Advancing The management of cow's milk protein allergy
- Extensively hydrolysed, lactose-free, hypoallergenic, whey-based formula for effective first-line relief from symptoms of cow’s milk protein allergy (CMPA) and/or severe gastrointestinal (GI) impairment (e.g. enteropathy)*1-6
- Contains 2’FL and LNnT† to nurture infants’ developing immune systems6-12
- Specifically designed to facilitate absorption and support gut recovery13-18
- Supports normal growth and development19
- Nutritionally complete. Suitable as a sole source of nutrition from birth or supplementary feeding from 6 months and up to 3 years of age
EFFECTIVELY RELIEVES SYMPTOMS, SUPPORTS GUT RECOVERY & GROWTH
Infants with CMPA may face an immediate challenge from skin, respiratory and/or gastrointestinal symptoms. These gastrointestinal symptoms can sometimes present as being severe (e.g. enteropathy).20-23 Alfaré® HMO is proven to be hypoallergenic* for infants with CMPA, with a very low allergenic profile, as a result of Nestlé Health Science’s state-of-the-art hydrolysis process.1-5 Alfaré® HMO effectively relieves infants from the symptoms of CMPA,*6 as well as promoting normal growth.19

Skin symptoms
(e.g. eczema, urticaria…)

Gastrointestinal symptoms
(e.g. vomiting, colic, diarrhoea…)
Respiratory symptoms
(e.g. wheezing…)
General symptoms
(e.g. inconsolable crying, failure to thrive…)
Alfaré® HMO is designed to support tissue repair and is proven to induce remission in infants with gastrointestinal disorders.13-18 This is due to it’s unique and lactose-free composition, including 40% easily absorbed medium-chain triglycerides (of total fat), 100% whey proteins and nucleotides.
Immune-nurturing benefits
CMPA is an immune-mediated disease. It is associated with gut microbiota dysbiosis, which impacts immune system maturation and leaves infants at an increased risk of infections and future allergies.25-33 Alfaré® HMO is the only lactose-free extensively hydrolysed formula to contain 2’-fucosyllactose (2’FL) and lacto-N-neotetraose (LNnT), which are structurally identical to two significant human milk oligosaccharides (HMO).†8-12
HMO support the growth of beneficial bacteria in the gut

HMO strengthen the gut barrier by promoting intestinal cell maturation

HMO eliminate pathogens through a decoy effect

HMO guide the maturation of the immune system, leading to a more balanced Th1/Th2 response
REDUCING INFECTIONS AND MEDICATION USE
Infants fed formula with 2’FL and LNnT† had fewer upper and lower respiratory tract infections6-8
Infants fed formula with 2’FL and LNnT† had lower antibiotic and antipyretic use 6-8
MEET EMMA
Feeding:
Exclusively formula-fed
Symptoms:
Severe diarrhoea indicating enteropathy, eczema, inconsolable crying
Diagnosis:
Suspected CMPA with enteropathy
Fictional patient profile for illustrative purpose only
WHAT WOULD BE YOUR MOST IMPORTANT GOAL FOR EMMA?
- SYMPTOM RELIEF
- GI REMISSION
- SUPPORT IMMUNE HEALTH
For infants with CMPA and severe gastrointestinal (GI) symptoms who cannot be breastfed, relief from allergy symptoms can be achieved by eliminating cow’s milk proteins from the infant’s diet with an appropriate hypoallergenic, specialty formula,20-22 like Alfaré® HMO.*2-6
Alfaré® HMO was shown to significantly reduce cow’s milk allergy symptoms scores (CoMiSS®):*
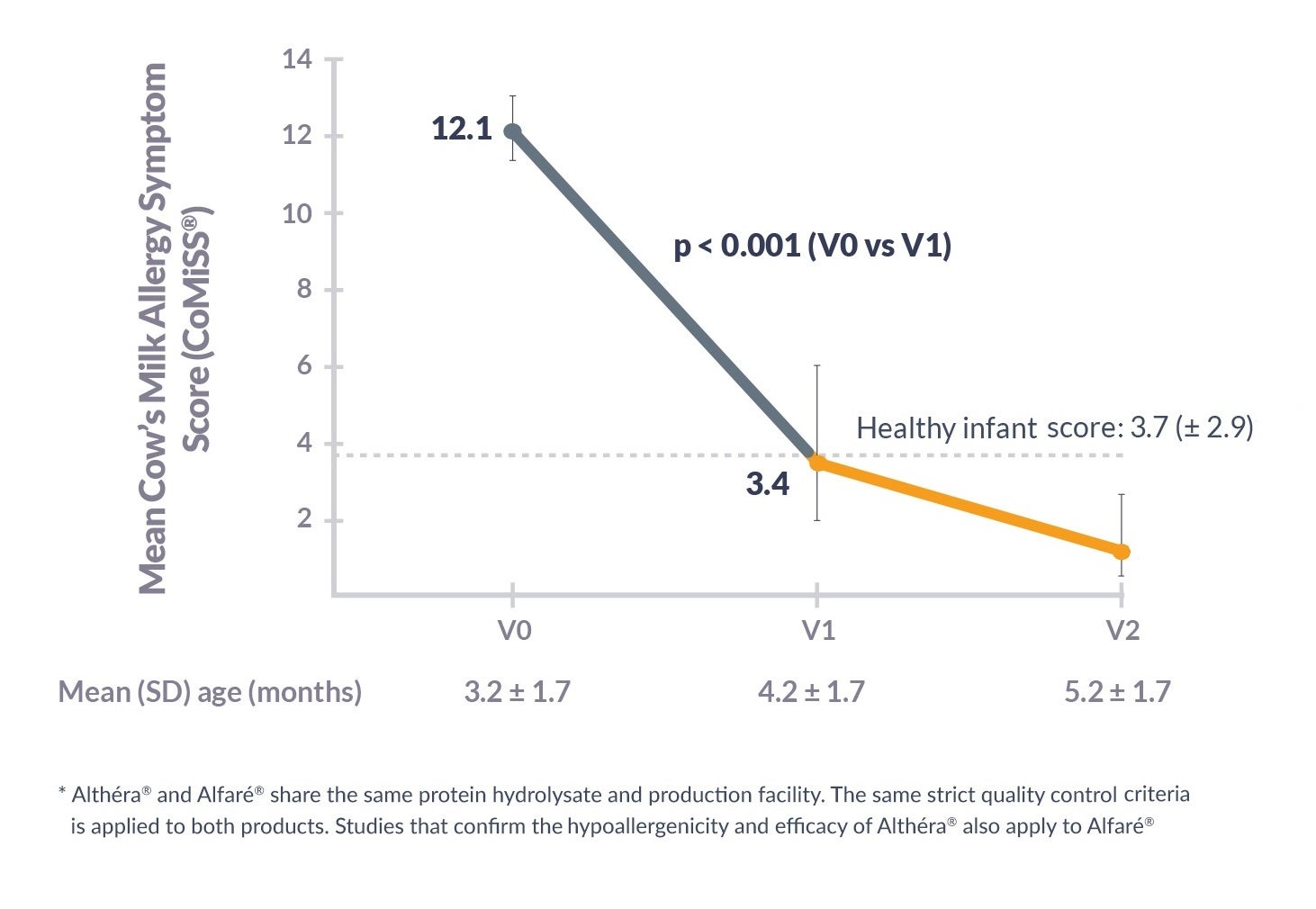
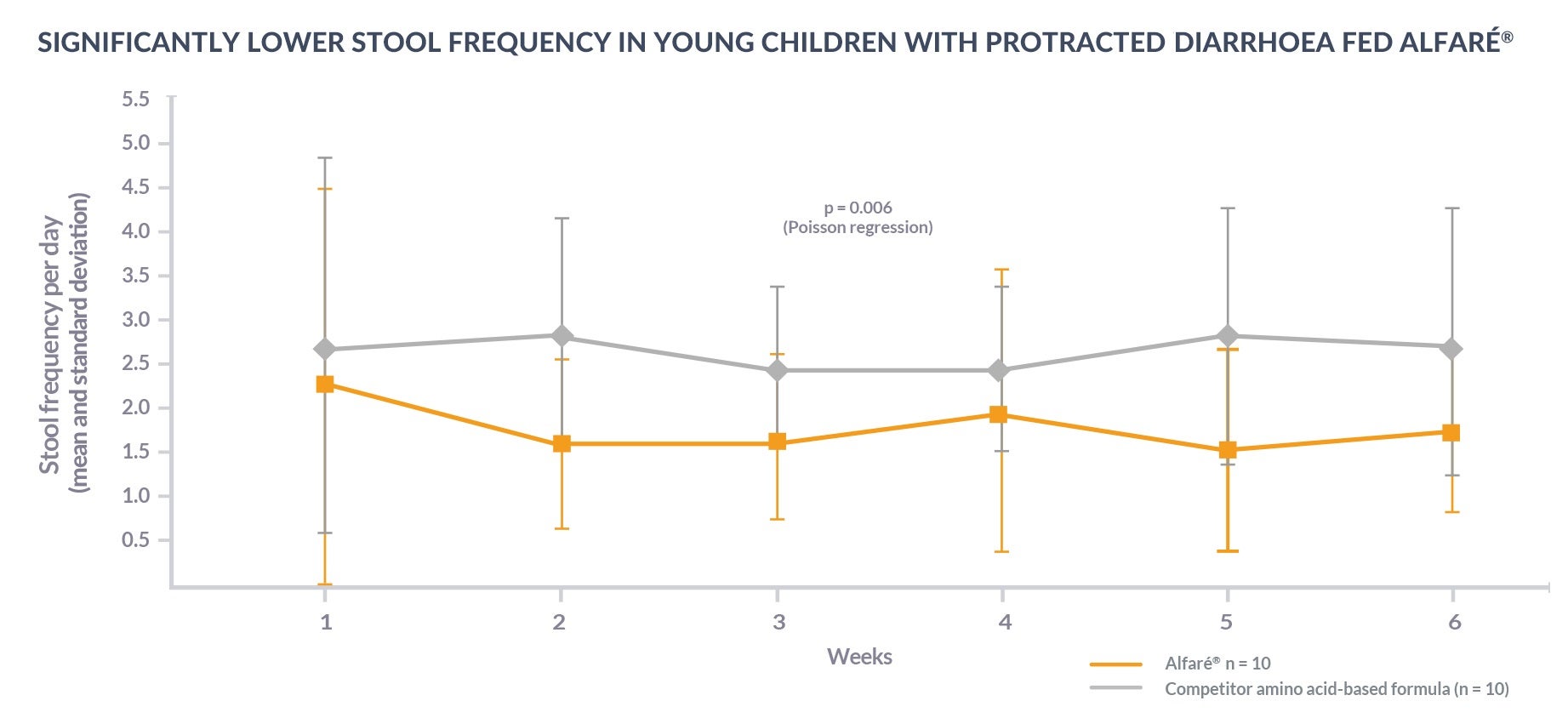
Alfaré® HMO is designed to supported gastrointestinal (GI) remission. Infants fed Alfaré® HMO were shown to have better control of protracted diarrhoea and reduced small intestinal mucosal inflammation after six weeks compared to a competitor amino acid-based formula:13
The development of the immune system of infants with CMPA is affected by gut microbiota dysbiosis and increased gut permeability.25-33
Alfaré® HMO contains 2’FL and LNnT to support the development of the immune system and reduce the risk of infections and associated medication use:6-8
| HEALTHY INFANTS8 Mean trial duration: 12 months |
INFANTS WITH CMPA6,7 Mean trial duration: 8.8 months |
|
|---|---|---|
| EAR INFECTIONS | -46%‡ | -71%‡ |
| LOWER RESPIRATORY TRACT INFECTIONS | -55%* | -34%‡ |
| BRONCHITIS | -70%** | |
| GASTROINTESTINAL INFECTIONS | -40%‡ | |
| FREQUENCY OF UPPER RESPIRATORY TRACT INFECTIONS | -42%* | |
| ANTIBIOTIC USE | -53%* | -6%‡ |
| ANTIPYRETIC USE | -56%*** | -11%‡ |
*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.05 at 4 months only; ‡ p = N.S.
FREQUENTLY ASKED QUESTIONS
Our range of immune-nurturing formulas for cow’s milk protein allergy
Our range of nutritional solutions are tailor-made for infants and young children with cow’s milk protein allergy and other food allergies/intolerances. The complete range has been designed to help manage cow’s milk protein allergy earlier, with the right product from the beginning.
*Althéra® HMO and Alfaré® HMO share the same protein hydrolysate and production facility. The same strict quality control criteria is applied to both products. Studies that confirm the hypoallergenicity and efficacy of Althéra® HMO also apply to Alfaré® HMO.
†Structurally identical Human Milk Oligosaccharides are not sourced from human milk.
REFERENCES
- Chauveau, A., et al. Immediate hypersensitivity to extensively hydrolysed formulas: An important reminder. Pediatric Allergy and Immunology, 2016. 27(5): p. 541-543.
- Niggemann, B., et al. Safety and efficacy of a new extensively hydrolysed formula for infants with cow’s milk protein allergy. Pediatr Allergy Immunol, 2008. 19(4): p. 348-54.
- Nowak-Wegrzyn A, et al. Confirmed Hypoallergenicity of a Novel Whey-Based Extensively Hydrolyzed Infant Formula Containing Two Human Milk Oligosaccharides Nutrients 2019;11(7):E1447.
- Nowak-Wegrzyn A, et al. Hypoallergenicity of a whey-based, extensively hydrolysed infant formula prepared with nonporcine enzymes. Allergy 2019;74(8):1582-1584.
- Nutten S, et al. Peptide size profile and residual immunogenic milk protein or peptide content in extensively hydrolysed infant formulas. Allergy, 2019.
- Nestlé Health Science, data on file. CINNAMON study.
- Vandenplas Y, et al. Growth, tolerance and safety of an extensively hydrolysed formula containing two human milk oligosaccharides in infants with cow’s milk protein allergy. Abstract presented at PAAM. Florence, Italy, October 19, 2019.
- Puccio G, et al. Effects of Infant Formula With Human Milk Oigosaccharides on Growth and Morbidity: A Randomized Multicenter Trial. J. Pediatr Gastroenterol Nutr 2017; 64(4):624-631.
- Bode L, Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology, 2012. 22(9): p. 1147-1162.
- Donovan SM and Comstock SS, Milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann Nutr Metab, 2016. 69(Suppl 2): p. 42-51.
- Sela DA and Mills DA, Nursing our microbiota: molecular linkages between bifidobacterial and milk oligosaccharides. Trends Microbiol, 2010. 18(7): p. 298-307.
- Ayechu-Muruzabal V, et al. Diversity of Human Milk Oligosaccharides and Effects on Early Life Immune Development. Front Pediatr 2018;6:239.
- Milla PJ, et al. A new semi-elemental diet for small intestinal inflammatory disease. Abstract P0583 presented at ESPGHAN, 2004. J Pediatr Gastroenterol Nutr 2004;39(Suppl 1).
- Schappi M, et al. Omega 3 PUFA enriched semi-elemental diet for protracted diarrhoea. Abstract PG3-14 presented at ESPGHAN, 2006.
- Delplanque B, et al. Lipid Quality in Infant Nutrition: Current Knowledge and Future Opportunities. J Pediatr Gastroenterol Nutr 2015;61(1):8-17.
- Mihatsch WA, et al. Hydrolysed protein accelerates the gastrointestinal transport of formula in preterm infants. Acta Paediatr 2001;90(2):196-198.
- Heine RG, et al. Lactose intolerance and gastrointestinal cow’s milk allergy in infants and children – common misconceptions revisited. World Allergy Organ J 2017;10(1):41.
- Carver JD. Dietary nucleotides: effects on the immune and gastrointestinal systems. Acta Pediatr Suppl 1999;88(430):83-88.
- Vandenplas Y, et al. Safety and adequacy of a semi-elemental formula for children with gastro-intestinal disease. Amino acids 2010;38(3):909-914.
- Koletzko S, et al. Diagnostic Approach and Management of Cow’s-Milk Protein Allergy in Infants and Children: ESPGHAN GI Committee Practical Guidelines. J Pediatr Gastroenterol Nutr 2012;55(2):221-229.
- Luyt D, et al. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin Exp Allergy 2014;44(5):642-672.
- Muraro A, et al. EAACI Food Allergy and Anaphyla,xis Guidelines: diagnosis and management of food allergy. Allergy 2014;69(8):1008–1025.
- Host A and Halken S. Cow's milk allergy: where have we come from and where are we going? Endocr Metab Immune Disord Drug Targets 2014;14(1):2-8.
- Nestlé Health Science, data on file. Alfaré® versus Neocate®, study No. 99.20.CLI.
- Azad MB, et al. Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy 2015;45(3):632-643.
- West CE, et al. The gut microbiota and its role in the development of allergic disease: a wider perspective Clin Exp Allergy 2015;45(1):43–53.
- Thompson-Chagoyan OC, et al. Faecal Microbiota and Short-Chain Fatty Acid Levels in Faeces from Infants with Cow‘s Milk Protein Allergy Int Arch Allergy Immunol 2011;156(3):325–332.
- Chin AM, et al. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin Cell Dev Biol 2017;66:81-93.
- Tanaka M and Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life Allergol Int 2017;66(4):515-522.
- Newburg DS and Walker WA. Protection of the Neonate by the Innate Immune System of Developing Gut and of Human Milk. Pediatr Res 2007;61(1):2-8.
- Woicka-Kolejwa K, et al. Food allergy is associated with recurrent respiratory tract infections during childhood. Postepy Dermatol Alergol 2016;33(2):109-113
- Juntti H, et al. Cow’s Milk Allergy is Associated with Recurrent Otitis Media During Childhood. Acta Otolaryngol 1999;119(8):867-873.
- Tikkanen S, et al. Status of children with cow’s milk allergy in infancy by 10 years of Age. Acta Paediatr 2000; 89(10):1174-1180.
- Azad MB et al. Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices J Nutr 2018;148(11):1733-1742.
- Petrus, N.C.M., Schoemaker, A.A., van Hoek, M.W. et al. Remaining symptoms in half the children treated for milk allergy. Eur J Pediatr 2015;174(6):759-765.
- Bach AC, et al. Medium-chain triglycerides: an update. Am J Clin Nutr 1982;36(5):950-962.
IMPORTANT NOTICE:
Mothers should be encouraged to continue breastfeeding even when their infants have cow’s milk protein allergy. This usually requires qualified dietary counseling to completely exclude all sources of cow’s milk protein from the mothers’ diet. If a decision to use a special formula intended for infants is taken, it is important to give instructions on correct preparation methods, emphasizing that unboiled water, unsterilized bottles or incorrect dilution can all lead to illness. Formula for special medical purposes intended for infants must be used under medical supervision.